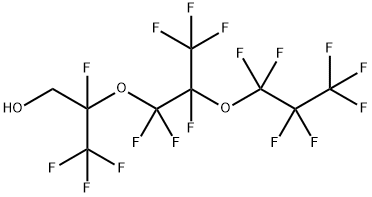
1H,1H-2,5-DI(TRIFLUOROMETHYL)-3,6-DIOXAUNDECAFLUORONONANOL synthesis
- Product Name:1H,1H-2,5-DI(TRIFLUOROMETHYL)-3,6-DIOXAUNDECAFLUORONONANOL
- CAS Number:14548-74-4
- Molecular formula:C9H3F17O3
- Molecular Weight:482.09
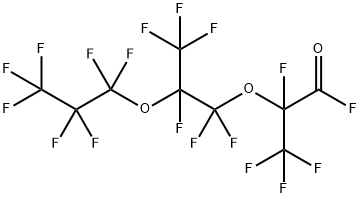
2641-34-1
141 suppliers
$45.00/1 g

14548-74-4
43 suppliers
$40.00/1 g
Yield:14548-74-4 90%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 20; for 2 h;Inert atmosphere;Reflux;
Steps:
1 Example 1
Approximately 1000 mL of water-free water (22.8 g, 0.6 mol) is placed in a 5 L dry bottle, under N2 protection, stirring and dropping hexane (250 g, 0.5 mol), Discharge, drop acceleration is maintainedAfter completion of the addition, stir at room temperature for 2 minutes.5 mL of Bulonic Acid500g ice water, re-join when release6N sulfuric acid oxidation for subsequent use,After the separation, the aqueous phase is 50mL x 3Incorporation of organic liquidWater-free sulfuric acid220g of steamed, rich,The yield is 90% and the GC ratio is 98.3%.1H NMR ,Chemistry 4.15: Multiple peaks, special CH2O peak.
References:
Shandong Dongyue Pending Qingneng Materials Co., Ltd.;Shi Dakuo;Feng Wei;Zhang Yongming;Rong Yue;Xu Yelin CN110790715, 2020, A Location in patent:Paragraph 0037-0039
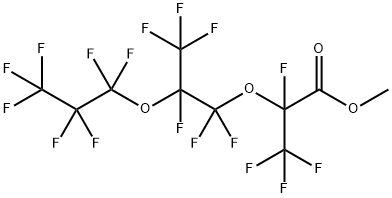
26131-32-8
75 suppliers
$29.19/1g

14548-74-4
43 suppliers
$40.00/1 g
![Methanesulfonic acid, 1,1,1-trifluoro-, 2,3,3,3-tetrafluoro-2-[1,1,2,3,3,3-hexafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)propoxy]propyl ester](/CAS/20210111/GIF/1173110-39-8.gif)
1173110-39-8
0 suppliers
inquiry

14548-74-4
43 suppliers
$40.00/1 g