(S)-2-((6-(3-aMinopiperidin-1-yl)-3-Methyl-2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)Methyl)benzonitrile synthesis
- Product Name:(S)-2-((6-(3-aMinopiperidin-1-yl)-3-Methyl-2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)Methyl)benzonitrile
- CAS Number:1353254-15-5
- Molecular formula:C18H25N3O2
- Molecular Weight:315.41
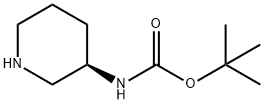
309956-78-3
575 suppliers
$11.00/5g
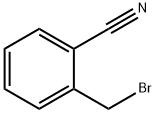
22115-41-9
456 suppliers
$5.00/5g

1353254-15-5
30 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate at 20; for 2.5 h;Sealed vial;
Steps:
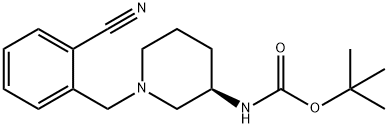
2-{[(3if)-3-aminopiperidin-l-yI]methy.}benzonitriIe Intermediate tert-but l [(3i?)-l-(2-cyanobenzyl)piperidiii-3-yl]carbamaie To a microwave vial equipped with a stir bar was added tert-butyl (3i?)-piperidin-3- ylcarbamate (3000 mg, 14.98 mmol), Potassium Carbonate (6211 mg, 44.9 mmol), and 2-(bromomethyl)benzonitrile (3230 mg, 1 .48 mmol). The vial was sealed and stirred at room temperature. The LCMS taken after 2.5 hours indicates the formation of the desired product. The crude reaction mixture was filtered and concentrated in vacuo. The material was dry loaded onto a 50 g column. The column was run from 100% dichloromethane to 15% methanol. The desired product eluted and the fractions were collected and concentrated in vacuo.
References:
WO2011/163330,2011,A1 Location in patent:Page/Page column 104-105