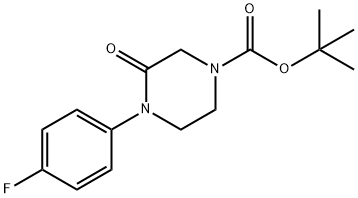
tert-Butyl 4-(4-fluorophenyl)-3-oxopiperazine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-fluorophenyl)-3-oxopiperazine-1-carboxylate
- CAS Number:1284243-44-2
- Molecular formula:C15H19FN2O3
- Molecular Weight:294.32
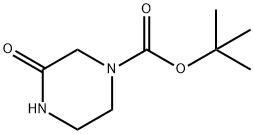
76003-29-7
244 suppliers
$5.00/5g
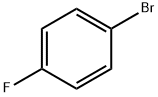
460-00-4
623 suppliers
$6.00/10g

1284243-44-2
27 suppliers
$29.00/100mg
Yield:1284243-44-2 73%
Reaction Conditions:
with copper(l) iodide;potassium carbonate;N,N`-dimethylethylenediamine in toluene;Inert atmosphere;Reflux;
Steps:
16.A
A mixture of 1 ,1-dimethylethyl 3-oxo-1-piperazinecarboxylate (0.200 g, 0.999 mmol), 1-bromo-4-fluorobenzene (0.091 mL, 0.832 mmol), copper(l) iodide (0.008 g, 0.042 mmol), potassium carbonate (0.230 g, 1.665 mmol) and N,N'-dimethyl-1 ,2- ethanediamine (0.009 mL, 0.083 mmol) in toluene (5 mL) was heated at reflux under nitrogen overnight. The reaction mixture was filtered through silica gel. The filtrate was evaporated and the residue was purified by silica gel chromatography (EtOAc:hexane) to give1 ,1-dimethylethyl 4-(4-fluorophenyl)-3-oxo-1-piperazinecarboxylate (0.180 g, 73%) as a white solid.
References:
WO2011/41713,2011,A2 Location in patent:Page/Page column 152

24424-99-5
840 suppliers
$13.50/25G

1284243-44-2
27 suppliers
$29.00/100mg