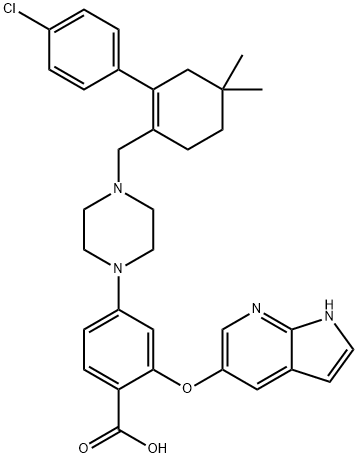
2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid synthesis
- Product Name:2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid
- CAS Number:1235865-77-6
- Molecular formula:C33H35ClN4O3
- Molecular Weight:571.11
![Methyl 2-[(1H-pyrrolo[2,3-b]pyridin-5-yl)oxy]-4-[4-[[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enyl]methyl]piperazin-1-yl]benzoate](/CAS/20180703/GIF/1235865-76-5.gif)
1235865-76-5
45 suppliers
inquiry
![2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid](/CAS/20180713/GIF/1235865-77-6.gif)
1235865-77-6
109 suppliers
inquiry
Yield: 96.9%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;methanol;water at 45;
Steps:
1.14 Step 14:2 - ((1H-pyrrolo [2,3-b] pyridin-5-yl) oxy) -4- (4 - ((4'-chloro-5,5-dimethyl- , 6-tetrahydro- [1,1'-biphenyl] -2-yl) methyl) piperazin-1-yl) benzoic acid
A solution of 2 - ((lH-pyrrolo [2,3-b] pyridin-5-yl) oxy) -4- (4 - ((4'-chloro-5,5-dimethyl- 2-yl) methyl) piperazin-1-yl) benzoate (1.65 g, 2.82 mmol, 1.0 eq)Was added to THF (30 mL) and methanol (10 mL), sodium hydroxide (0.60 g, 15.0 mmol, 5.5 eq) and water (6 mL) were added and reacted at 45 ° C overnight. Point half analysis, the raw material has been completely responsive. After cooling, the mixture was poured into 100 mL of water, adjusted to pH = 2 with 2M hydrochloric acid and extracted with DCM (30 mL x 3). The organic phase was dried over anhydrous sodium sulfate, spin dried, and then 5 mL of DCM and 100 mL of PE were stirred for 10 min , Filtered to filter the white solid product 1.56g, the yield 96.9%.
References:
Sunshine Lake Pharma Co.,Ltd.;Kou, Yuhui;Hu, Bolin;Jiang, Haigang;Ye, Jiuyong;Liu, Zhiqiang;Xie, Hongming;Zhang, Yingjun CN106565706, 2017, A Location in patent:Paragraph 0209; 0210; 0211
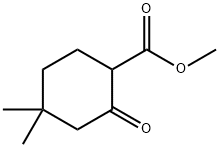
32767-46-7
36 suppliers
$120.00/50mg
![2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid](/CAS/20180713/GIF/1235865-77-6.gif)
1235865-77-6
109 suppliers
inquiry
![Methyl 4,4-dimethyl-2-[(trifluoromethylsulfonyl)oxy]cyclohex-1-ene-1-carboxylate](/CAS/20180703/GIF/1228780-46-8.gif)
1228780-46-8
7 suppliers
inquiry
![2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid](/CAS/20180713/GIF/1235865-77-6.gif)
1235865-77-6
109 suppliers
inquiry
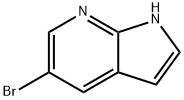
183208-35-7
561 suppliers
$5.00/1g
![2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid](/CAS/20180713/GIF/1235865-77-6.gif)
1235865-77-6
109 suppliers
inquiry
![5-BROMO-1-TRIISOPROPYLSILANYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/858116-66-2.gif)
858116-66-2
75 suppliers
$55.00/250mg
![2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid](/CAS/20180713/GIF/1235865-77-6.gif)
1235865-77-6
109 suppliers
inquiry