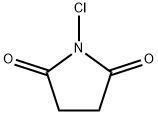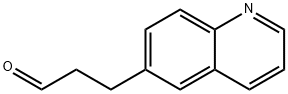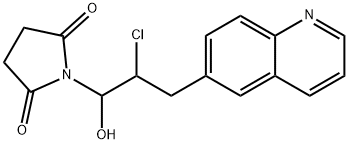
1-[2-Chloro-1-hydroxy-3-(6-quinolinyl)propyl]-2,5-pyrrolidinedione synthesis
- Product Name:1-[2-Chloro-1-hydroxy-3-(6-quinolinyl)propyl]-2,5-pyrrolidinedione
- CAS Number:1197377-31-3
- Molecular formula:C16H15ClN2O3
- Molecular Weight:318.75
![1-[2-Chloro-1-hydroxy-3-(6-quinolinyl)propyl]-2,5-pyrrolidinedione 1-[2-Chloro-1-hydroxy-3-(6-quinolinyl)propyl]-2,5-pyrrolidinedione](/NewsImg/2023-11-13/6383548140088645036596909.jpg)
Yield: 69%
Reaction Conditions:
with benzoic acid;L-proline in acetonitrile at 0 - 20;Product distribution / selectivity;
Steps:
18.II 1-(2-Chloro-1-hydroxy-3-(quinolin-6-yl)propyl)pyrrolidine-2,5-dione (11)
Method II. A solution of 3-quinolin-6-ylpropanal (9, 74.8 g, 0.404 mol) in acetonitrile (202 mL, 3.87 mol) was cooled to 0° C. before L-proline (4.70 g, 0.0404 mol, 0.10 equiv), benzoic acid (4.96 g, 0.0404 mol, 0.10 equiv), and N-chlorosuccinimide (NCS, 57.8 g, 0.424 mol, 1.05 equiv) were added at 0° C. The reaction mixture was stirred at 0° C. for 3 h and the resulting clear solution was allowed to warm to room temperature and stirred at room temperature for 18 h. The reaction mixture became a thick suspension and LCMS showed the completion of the reaction. Ethyl acetate (EtOAc, 202 mL) was added to the reaction mixture and the resulting mixture was stirred at room temperature for 1 h. The solids were collected by filtration, washed with ethyl acetate (EtOAc, 100 mL) and dried under vacuum at 40-45° C. to constant weight to afford 1-(2-chloro-1-hydroxy-3-(quinolin-6-yl)propyl)pyrrolidine-2,5-dione (11, 88.8 g, 128.8 g theoretical, 69% yield) as an off-white powder, which was found to be identical to the material made from method I in every comparable aspect.
References:
Weng, Lingkai;Qiao, Lei;Zhou, Jiacheng;Liu, Pingli;Pan, Yongchun US2009/291956, 2009, A1 Location in patent:Page/Page column 22; 23-24
5332-25-2
419 suppliers
$6.00/5g
![1-[2-Chloro-1-hydroxy-3-(6-quinolinyl)propyl]-2,5-pyrrolidinedione](/CAS/20150408/GIF/1197377-31-3.gif)
1197377-31-3
49 suppliers
inquiry
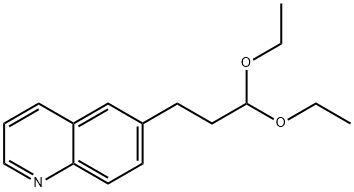
1197377-26-6
1 suppliers
inquiry
![1-[2-Chloro-1-hydroxy-3-(6-quinolinyl)propyl]-2,5-pyrrolidinedione](/CAS/20150408/GIF/1197377-31-3.gif)
1197377-31-3
49 suppliers
inquiry