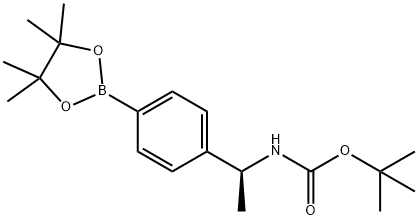
Carbamic acid, N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]-, 1,1-dimethylethyl ester
- CAS Number:1171897-03-2
- Molecular formula:C19H30BNO4
- Molecular Weight:347.26
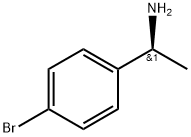
![(S)-[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/847728-89-6.gif)
847728-89-6
104 suppliers
$45.00/50mg
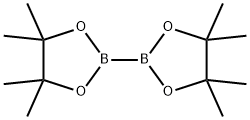
73183-34-3
583 suppliers
$6.00/5g
![Carbamic acid, N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1171897-03-2.gif)
1171897-03-2
27 suppliers
inquiry
Yield:1171897-03-2 99%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 90; for 2 h;Temperature;
Steps:
1 Exemplary Synthesis of Exemplary Compound 167 Step 1
To a solution of tert-butyl N-[(1S)-1-(4-bromophenyl)ethyl]carbamate (5 g, 16.7 mmol, 1 eq) and 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolane (5.08 g, 20.0 mmol, 1.2 eq) in dioxane (100 mL) was added [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (975 mg, 1.33 mmol, 0.08 eq) and potassium acetate (3.27 g, 33.31 mmol, 2 eq).
The mixture was stirred at 90° C. for 2 hours.
The reaction mixture was concentrated to give crude product.
The crude product was purified by silica gel chromatography (petroleum ether:ethyl acetate=1:0 to 10:1).
Compound tert-butyl N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]carbamate (8.2 g, 16.44 mmol, 99% yield) was obtained as a yellow oil.
References:
US2019/300521,2019,A1 Location in patent:Paragraph 1203-1205

24424-99-5
846 suppliers
$13.50/25G
![Carbamic acid, N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1171897-03-2.gif)
1171897-03-2
27 suppliers
inquiry

4187-56-8
121 suppliers
$8.00/250mg
![Carbamic acid, N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1171897-03-2.gif)
1171897-03-2
27 suppliers
inquiry
27298-97-1
256 suppliers
$9.00/1g
![Carbamic acid, N-[(1S)-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1171897-03-2.gif)
1171897-03-2
27 suppliers
inquiry
