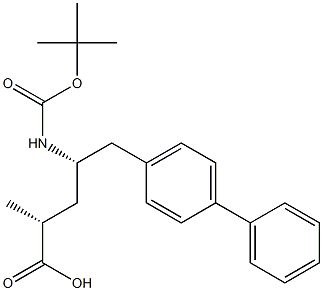
(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid synthesis
- Product Name:(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid
- CAS Number:1012341-50-2
- Molecular formula:C23H29NO4
- Molecular Weight:383.48
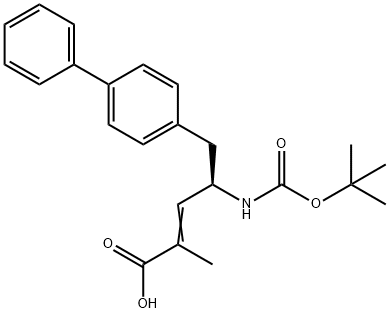
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
224 suppliers
$9.00/1g
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid](/CAS/20150408/GIF/1012341-50-2.gif)
1012341-50-2
420 suppliers
$5.00/100mg
Yield:1012341-50-2 91.9%
Reaction Conditions:
with ammonium formate;nickel(II) acetate tetrahydrate;(R,R)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine in ethanol at 55; for 8 h;Temperature;
Steps:
1-9 Example 9The compound (2R, 4S)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpentanoic acid of this example is prepared as follows
Under an atmosphere of air, place the compound of formula II (E)-(R)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid in a clean reaction flask 38.1g (0.1 mol),Nickel acetate tetrahydrate 373.5mg (1.5mmol),(R,R)-(-)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine 815mg (1.5mmol) and ammonium formate 37.8g ( 0.6mol) dissolved in 500mL ethanol solvent, stirred and mixed well, and heated up,The mixture was stirred at 55° C. for an asymmetric reduction reaction for 8 hours,After the reaction, ethanol was removed by distillation under reduced pressure,To the residue, add 30 g of diatomaceous earth and 300 mL of isopropyl acetate,After stirring for 1 hour and filtering, the resulting filtrate was heated to reflux,800 mL of n-heptane was added dropwise within 2 hours, and then slowly cooled to room temperature,When the cooling rate is 5/hour, a large number of crystals are precipitated.The resulting crystals were filtered and dried under vacuum at 40°C,The compound of formula I is obtained as a white solid product(2R, 4S)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpentanoic acid 35.1g,The yield was 91.9%.
References:
Taizhou Polytechnic College;Zhou Liping;Chen Yunhua;Ye Haiwei;Jiang Ying;Lu Congling;Hu Meijie CN111269148, 2020, A Location in patent:Paragraph 0033-0061
149709-68-2
3 suppliers
inquiry
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid](/CAS/20150408/GIF/1012341-50-2.gif)
1012341-50-2
420 suppliers
$5.00/100mg
149709-61-5
1 suppliers
inquiry
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid](/CAS/20150408/GIF/1012341-50-2.gif)
1012341-50-2
420 suppliers
$5.00/100mg
![1-Pyrrolidinecarboxylic acid, 5-([1,1'-biphenyl]-4-ylmethyl)-3-methyl-2-oxo-, 1,1-dimethylethyl ester, (3R,5S)-](/CAS/20210305/GIF/1038924-76-3.gif)
1038924-76-3
2 suppliers
inquiry
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid](/CAS/20150408/GIF/1012341-50-2.gif)
1012341-50-2
420 suppliers
$5.00/100mg
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-aMino-2-Methylpentanoic acid hydrochloride](/CAS/20150408/GIF/1038924-71-8.gif)
1038924-71-8
63 suppliers
inquiry

24424-99-5
863 suppliers
$13.50/25G
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid](/CAS/20150408/GIF/1012341-50-2.gif)
1012341-50-2
420 suppliers
$5.00/100mg