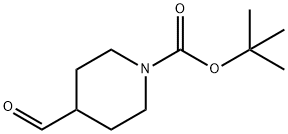
1-tert-Butoxycarbonyl-4-piperidinecarboxaldehyde synthesis
- Product Name:1-tert-Butoxycarbonyl-4-piperidinecarboxaldehyde
- CAS Number:137076-22-3
- Molecular formula:C11H19NO3
- Molecular Weight:213.27
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
186 suppliers
$6.00/100mg

137076-22-3
366 suppliers
$5.00/1g
Yield:137076-22-3 100%
Reaction Conditions:
Stage #1: tert-butyl 4-(methoxy(methyl)carbamoyl)piperidine-1-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at -10; for 0.166667 - 0.333333 h;
Stage #2: with hydrogenchloride;potassium hydrogensulfate in tetrahydrofuran;water; for 0.25 h;
Steps:
3
To a stirred solution of the above Weinreb amide (20) (2.70 g, 10 mmol) in THF (100 mL) at -10° C. was added LiAlH4 (460 mg, 12 mmol). After the starting material was consumed completely (10-20 min), a solution of KHSO4 (2.8 g) in H2O (100 mL) was added slowly, then followed by 1 N HCl (50 mL). The mixture was stirred for 15 minutes and extracted with EtOAc (3×100 mL). The combined organic extracts were washed with sat NaHCO3 (50 mL), brine (50 mL), dried (MgSO4), and concentrated to give an oil (21) (2.14 g, 100%). 1H NMR (500 MHz, CDCL3) δ9.66 (s, 1H), 3.98 (br d, J=11.5 Hz, 2H), 2.93 (m, 2H), 2.41 (m, 1H), 1.90 (m, 2H), 1.55 (m, 2H), 1.46 (s, 9H); MS (ESI+) m/z 158.67 (M+H+-isobutylene).
References:
US2004/14763,2004,A1 Location in patent:Page 21
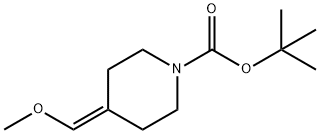
138022-91-0
1 suppliers
inquiry

137076-22-3
366 suppliers
$5.00/1g
![Tert-Butyl 4-[Methoxy(Methyl)Carbamoyl]Piperidine-1-Carboxylate](/CAS/20200119/GIF/370864-67-8.gif)
370864-67-8
5 suppliers
inquiry

137076-22-3
366 suppliers
$5.00/1g
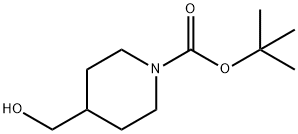
123855-51-6
427 suppliers
$6.00/5g

137076-22-3
366 suppliers
$5.00/1g
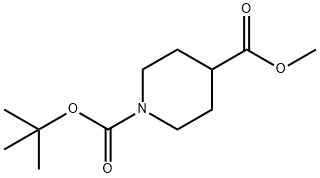
124443-68-1
266 suppliers
$6.00/5g

137076-22-3
366 suppliers
$5.00/1g