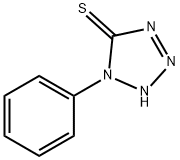
1-Phenyltetrazole-5-thiol synthesis
- Product Name:1-Phenyltetrazole-5-thiol
- CAS Number:86-93-1
- Molecular formula:C7H6N4S
- Molecular Weight:178.21
103-72-0
239 suppliers
$10.00/1g

86-93-1
426 suppliers
$10.00/1g
Yield:86-93-1 99%
Reaction Conditions:
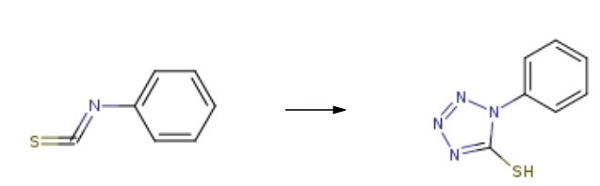
with sodium azide;zinc(II) chloride in acetonitrile at 80; for 1 h;
Steps:
1-Allyl-1H-tetrazole-5-thiol (4a).
General procedure: Sodium azide, 0.79 g (12.1 mmol), and 1.65 g (12.1 mmol) of ZnCl2 were added to 30 mL of MeCN. The mixture was heated with stirring until boiling (80 °C), and then 1 g (10.1 mmol) of allyl isothiocyanate was added. The reaction mixture was stirred for 1 h at 80 °C, and the solvent was then removed in a vacuum. The residue was treated with 5% aqueous NaOH (50 mL) with stirring for 20 min. The suspension was filtered, and the filtrate was treated with chloroform (2×10 mL) to remove impurities. The aqueous layer was acidified with conc. HCl to pH 1. The precipitate that formed was filtered off, washed with water, and dried in air. Compounds 4b-4f were prepared in a similar way. 1-Phenyl-1H-tetrazole-5-thiol (4c). Yield 1.31 g(99%), colorless crystals, mp 148-150 °C (H2O) (mp 150 °C [14]). IR spectrum, ν, cm-1: 2549 (SH), 1510 (C=N), 1457 (Ph), 1153 (C-N), 725 (C-S). 1H NMR spectrum, δ, ppm: 7.44-7.54 m (3, H-Ph), 7.89-7.92m (2H, -Ph). 13C NMR spectrum, δ, ppm: 124.0 (Ph), 129.3 (Ph), 134.8 (Ph), 164.1 (C-S). Found, %: C47.29; H 3.50; N 31.56; S 18.11. C7H6N4S. Calculated,%: C 47.18; H 3.39; N 31.44; S 17.99.
References:
Myznikov;Vorona;Artamonova;Zevatskii, Yu. E. [Russian Journal of General Chemistry,2017,vol. 87,# 4,p. 731 - 738][Zh. Obshch. Khim.,2017,vol. 87,# 4,p. 597 - 604,8]
18233-34-6
3 suppliers
inquiry

86-93-1
426 suppliers
$10.00/1g
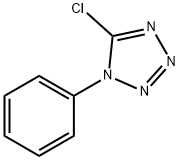
14210-25-4
84 suppliers
$17.67/250mgs:

86-93-1
426 suppliers
$10.00/1g
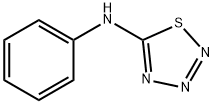
13078-30-3
10 suppliers
inquiry

86-93-1
426 suppliers
$10.00/1g
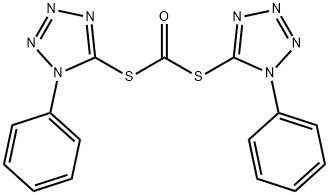
32276-00-9
4 suppliers
inquiry
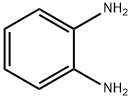
95-54-5
516 suppliers
$9.00/1g
615-16-7
392 suppliers
$8.00/10g

86-93-1
426 suppliers
$10.00/1g