
1-Bromooctane synthesis
- Product Name:1-Bromooctane
- CAS Number:111-83-1
- Molecular formula:C8H17Br
- Molecular Weight:193.12

111-87-5
777 suppliers
$6.00/100g

111-83-1
504 suppliers
$12.00/25g
Yield:111-83-1 98%
Reaction Conditions:
with sulfuric acid;hydrogen bromide in lithium hydroxide monohydrate at 60;Flow reactor;Green chemistry;Temperature;
Steps:
1.1; 1.2; 1.3; 1.4; 1.5; 2.1; 2.2; 2.3; 2.4; 2.5; 3.1; 3.2; 3.3; 3.4; 3.5
(1) The molar ratio of n-octanol to hydrobromic acid is 1: 1.1, that is, take 41.2g of n-octanol and 70g of 40% hydrobromic acid into two containers;(2) The molar ratio of n-octanol to concentrated sulfuric acid is 1: 0.8, that is, take 26g of concentrated sulfuric acid and hydrobromic acid and mix them in the microreactor;(3) Use a constant flow pump to pump concentrated sulfuric acid and hydrobromic acid into the microreactor, and then pump n-octanol to control the flow rate of the pumped solution to 0.4 mL / min;(4) maintaining the reaction temperature at 60 ° C;(5) Dilute the collected solution with water, separate the organic layer, and obtain a 1-bromo-n-octane yield of 98% after purification.
References:
CN110655445,2020,A Location in patent:Paragraph 0017-0037

111-66-0
171 suppliers
$10.00/10ml

111-83-1
504 suppliers
$12.00/25g
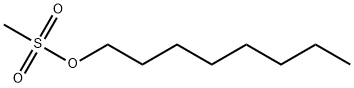
16156-52-8
2 suppliers
inquiry

111-83-1
504 suppliers
$12.00/25g

463-11-6
52 suppliers
$25.00/1g

111-83-1
504 suppliers
$12.00/25g
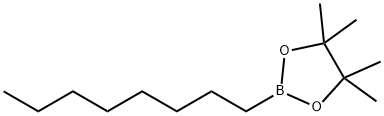
66217-56-9
17 suppliers
inquiry

111-83-1
504 suppliers
$12.00/25g