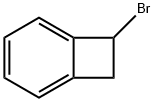
1-Bromobenzocyclobutene synthesis
- Product Name:1-Bromobenzocyclobutene
- CAS Number:21120-91-2
- Molecular formula:C8H7Br
- Molecular Weight:183.05
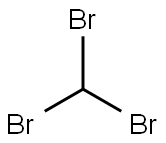
75-25-2
345 suppliers
$16.00/50g
544-25-2
117 suppliers
$10.00/5g

21120-91-2
90 suppliers
$30.00/100mg
Yield: 5.94%
Reaction Conditions:
with 18-crown-6 ether;potassium carbonate at 145; for 9 - 10 h;
Steps:
45
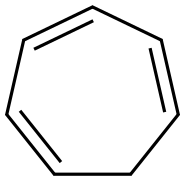
In a 250 mL round bottomed flask equipped with a drying tube and a condenser, a solution of cycloheptatriene (30.7 g, 300 mmol), bromoform (25.3 g, 100 mmol), anhydrous K2CO3 (15.0 g, 109 mmol), and 18-crown-6 (0.75 g) was heated with stirring at 145 C for 9-10 h. The solution was allowed to cool and diluted with an equal15 volume of acetone. Silica gel (15.0 g) was added to reaction mixture and the insoluble solid residue was separated via vacuum filtration and the filter cake washed with acetone until the washings were colorless. The filtrate was concentrated and distilled to remove residual cycloheptatriene. The viscous, brown residue was precipitated into hot petroleum ether. After filtration to remove the precipitate, the filtrate was concentrated and distilled in vacuo20 through a Vigreaux column to give slightly impure product. Pure 1 -bromobenzocyclobutene was obtained as a light yellow liquid by redistillation yield (2.95 g, 5.94 %). 1H NMR (300 MHz, CDCl3) δ 7.28 (m, IH, ArH), 7.16 (d, IH, J=7.0Hz, ArH), 7.07 (d, IH, J=6.4Hz, ArH), 5.39 (m, IH, CH), 3.85 (dd, IH, J=4.4Hz, J=14.7Hz, CH2), 3.45 (d, IH, J=14.7Hz, CH2).
References:
VANDERBILT UNIVERSITY WO2008/24435, 2008, A2 Location in patent:Page/Page column 104
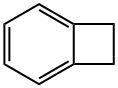
694-87-1
160 suppliers
$22.00/250mg

21120-91-2
90 suppliers
$30.00/100mg

75-25-2
345 suppliers
$16.00/50g

544-25-2
117 suppliers
$10.00/5g

21120-91-2
90 suppliers
$30.00/100mg
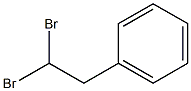
2612-38-6
0 suppliers
inquiry
![Bicyclo[4.2.0]octa-1,3,5-triene,7,8-diiodo-](/CAS/20180601/GIF/6639-21-0.gif)
6639-21-0
0 suppliers
inquiry

21120-91-2
90 suppliers
$30.00/100mg
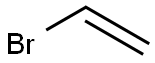
593-60-2
145 suppliers
$25.00/25g
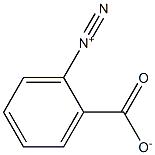
1608-42-0
1 suppliers
inquiry

21120-91-2
90 suppliers
$30.00/100mg