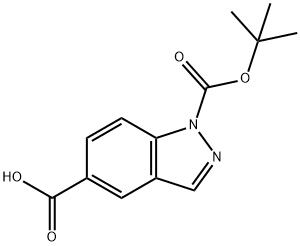
1-BOC-5-INDAZOLECARBOXYLIC ACID synthesis
- Product Name:1-BOC-5-INDAZOLECARBOXYLIC ACID
- CAS Number:885954-14-3
- Molecular formula:C13H14N2O4
- Molecular Weight:262.26
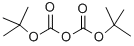
24424-99-5
861 suppliers
$13.50/25G
61700-61-6
221 suppliers
$7.00/250mg

885954-14-3
32 suppliers
$183.59/250mg
Yield:885954-14-3 40%
Reaction Conditions:
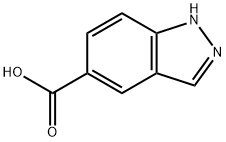
Stage #1: di-tert-butyl dicarbonate;1H-indazole-5-carboxylic acidwith sodium hydroxide in 1,4-dioxane;lithium hydroxide monohydrate at 20; for 16 h;Inert atmosphere;
Stage #2: with citric acid in lithium hydroxide monohydrate; pH=~ 4;
Steps:
24
Preparation 24: 1 -(terf-butoxycarbonyl)-1 H-indazole-5-carboxylic acid; To a suspension of 1 H-indazole-5-carboxylic acid (9g, 0.055mol) in 1 ,4-dioxane (200ml) was added aqueous NaOH (66ml, 1 M, 0.066mol), followed by di-ferf-butyldicarbonate (13.2g, 0.06mol). The mixture was stirred at room temperature under nitrogen for 16 hours and turned from a clear to cloudy mixture. The reaction mixture was concentrated in vacuo, and the pH adjusted to -4 by addition of 10% aqueous citric acid solution, which resulted in formation of a cream coloured solid. The solid was isoalted by filtration, washed with water, then dissolved in acetone, dried over Na2S04, filtered and evaporated to give the crude product (1 Og) as a light yellow solid. Purification by column chromatography (eluting with petroleum ether/EtOAc from 20:1 to 5: 1 ) gave the title compound as an off-white solid (4g, 40%) and recovered 1 H-indazole-5-carboxylic acid (4g, 44%).1H NMR (400 MHz, CDCI3) δ ppm 1.74 (s, 9H) 8.28 (d, 2H) 8.30 (s, 1 H) 8.58 (s, 1 H)
References:
WO2012/95781,2012,A1 Location in patent:Page/Page column 100-101