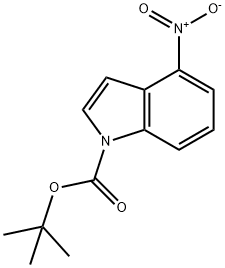
1-BOC-4-NITROINDOLE synthesis
- Product Name:1-BOC-4-NITROINDOLE
- CAS Number:913836-24-5
- Molecular formula:C13H14N2O4
- Molecular Weight:262.26
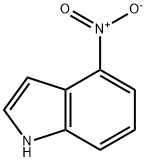
4769-97-5
350 suppliers
$6.00/250mg

24424-99-5
864 suppliers
$13.50/25G

913836-24-5
59 suppliers
$22.00/250mg
Yield:913836-24-5 95.8%
Reaction Conditions:
dmap in toluene at 20; for 1.75 h;Product distribution / selectivity;
Steps:
1
Step 1: Preparation of 4-Nitro-indole-1-carboxylic acid tert-butyl ester (2) To a 5 L four-necked round bottom flask equipped with mechanical stirrer, nitrogen inlet, thermocouple, and condenser was charged 182.3 g (1.124 mol) of 4-nitroindole, 1400 mL toluene, and 2.7 g of DMAP. At room temperature, solid BOC2O (270.0 g 1.237 mol, 1.10 eq) was added in portions over about 45 minutes to the stirring mixture while maintaining moderate gas evolution. After stirring the resulting solution for 1 hour, HPLC analysis showed the reaction to be complete with no starting material. The batch was quenched with 500 mL water, transferred to a separatory funnel and the phases were split. The organic layer was washed with 500 mL brine, concentrated to a slurry, diluted with 1000 ml heptane, filtered, and washed with fresh heptane. The filter cake was dried under vacuum at RT to constant weight to afford 206.0 gram of pale yellow solid. A second crop of 20.0 gram of product was isolated as post-precipitate from the mother liquors. The overall yield was 226.0 gram, 95.8% yield of pale yellow solid (100 area % HPLC).
References:
US2010/36123,2010,A1 Location in patent:Page/Page column 4; 5; 7; 8