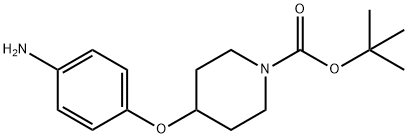
1-BOC-4-(4-AMINO-PHENOXY)-PIPERIDINE synthesis
- Product Name:1-BOC-4-(4-AMINO-PHENOXY)-PIPERIDINE
- CAS Number:138227-63-1
- Molecular formula:C16H24N2O3
- Molecular Weight:292.37
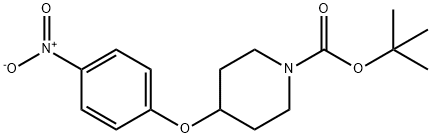
138227-62-0
53 suppliers
$75.00/100mg

138227-63-1
100 suppliers
$45.00/50mg
Yield:138227-63-1 99%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol at 20; for 4 h;
Steps:
106 Reference example 106; 4-(1-t-Butoxycarbonylpiperidin-4-yloxy)aniline
To a solution of 4-(1-t-butoxycarbonylpiperidin-4-yloxy)nitrobenzene (11.9 g) obtained in reference example 105 in methanol (100 ml) was added palladium on carbon (1.9 g), and the resulting mixture was stirred at room temperature under a hydrogen atmosphere for 4 hours. At the end of this time, the reaction mixture was filtered, and the filtrate was evaporated in vacuo. The residue obtained was purified by chromatography on a silica gel column using a mixed solvent of hexane and ethyl acetate (1:1) as the eluent to afford the title compound (10.7 g, yield: 99 %) as a pale red solid. 1H NMR (400MHz, CDCl3) δ ppm : 1.46 (9H, s), 1.71 (2H, m), 1.87 (2H, m), 3.27 (2H, m), 3.71 (2H, m), 4.26 (1H, m), 6.63 (2H, d, J=8.5), 6.76 (2H, d, J=8.5).
References:
EP1375482,2004,A1 Location in patent:Page 159
141-78-6
653 suppliers
$22.37/250ml

138227-62-0
53 suppliers
$75.00/100mg

138227-63-1
100 suppliers
$45.00/50mg
350-46-9
526 suppliers
$10.60/1gm:

138227-63-1
100 suppliers
$45.00/50mg
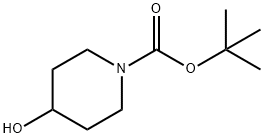
109384-19-2
452 suppliers
$5.00/1g

138227-63-1
100 suppliers
$45.00/50mg
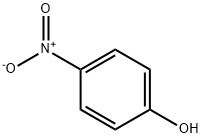
100-02-7
36 suppliers
$11.00/5G

138227-63-1
100 suppliers
$45.00/50mg