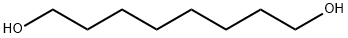
1,8-Octanediol synthesis
- Product Name:1,8-Octanediol
- CAS Number:629-41-4
- Molecular formula:C8H18O2
- Molecular Weight:146.23
505-48-6
435 suppliers
$8.00/10g
629-41-4
376 suppliers
$9.00/10g
Yield:629-41-4 94.8 %Chromat.
Reaction Conditions:
with cobalt(II) oxide;hydrogen in 1,4-dioxane at 189.84; under 30003 Torr; for 10 h;Autoclave;
Steps:
2.3 Catalytic evaluation and product analysis
General procedure: The hydrogenation of carboxylic acids or other substrates was performed in a high-pressure stainless-steel autoclave (Xinyuan Chemical Machinery, Series CJK, 300 mL) with a maximum stirring rate of 1500 r/min. In a typical experiment, 0.2 g of catalyst (or without catalyst for the control experiment), 3 mmol of the substrate, and 100 mL alkane solvent (n-hexane, n-heptane, i-octane, or n-dodecane) were well mixed in the autoclave and purged with pure nitrogen at room temperature. The gas supply and discharge were carried out manually through needle valves. The autoclave was rapidly heated to the desired temperature and hydrogen was introduced at 2 MPa to initiate the reaction. The reaction pressure was kept at 2 MPa with a small negative deviation (~0.2 MPa) owing to the consumption of hydrogen. Samples of the liquid phase were continuously taken through a sampling tube with a filter at certain intervals. The stirring rate was kept at 750 r/min during the reaction.
References:
Song, Song;Wang, Dong;Di, Lu;Wang, Chuanming;Dai, Weili;Wu, Guangjun;Guan, Naijia;Li, Landong [Cuihua Xuebao/Chinese Journal of Catalysis,2018,vol. 39,# 2,p. 250 - 257]

50816-19-8
199 suppliers
$5.00/250mg
629-41-4
376 suppliers
$9.00/10g

22054-14-4
4 suppliers
inquiry
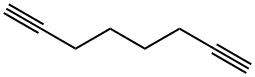
871-84-1
132 suppliers
$45.00/50mg
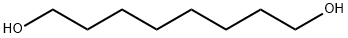
629-41-4
376 suppliers
$9.00/10g
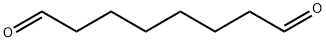
638-54-0
13 suppliers
inquiry
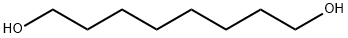
629-41-4
376 suppliers
$9.00/10g
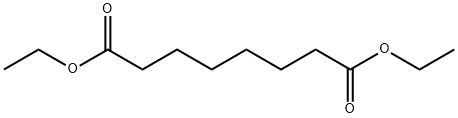
2050-23-9
190 suppliers
$60.00/25g
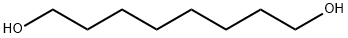
629-41-4
376 suppliers
$9.00/10g