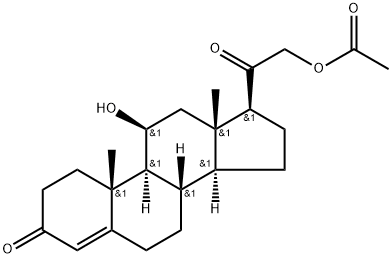
CORTICOSTERONE 21-ACETATE synthesis
- Product Name:CORTICOSTERONE 21-ACETATE
- CAS Number:1173-26-8
- Molecular formula:C23H32O5
- Molecular Weight:388.5
50-22-6
241 suppliers
$23.00/100mg
108-24-7
5 suppliers
$14.00/250ML

1173-26-8
71 suppliers
$45.00/25mg
Yield:1173-26-8 75%
Reaction Conditions:
with pyridine at 20;
Steps:
2 2. Synthesis of A2
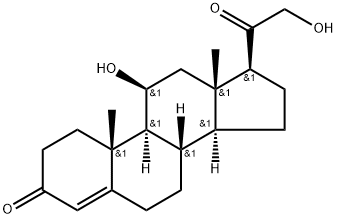
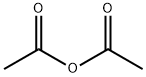
2. Synthesis of A2Into a 250-mL 3-necked round-bottom flask, was placed Al, (8S,9S,1OR,i iS, i35,i45, i75)- i i-hydroxy-i7-(2-hydroxyacetyl)- i0,i3-dimethyl-i ,7,8, i 0, ii, i2, i 3, i 5, i 6, i7-decahydro-2H-cyclopenta[a]phenanthren-3(6H,9H, i4H)-one (9.9 g, 28.57 mmol, i.00 equiv), Pyridine (i iO mL), Ac20 (4.4 g). The resulting solution was stirred for i overnight at room temperature. The resulting mixture was concentrated under vacuum. The resulting solution was extracted with 3x300 mL of ethyl acetate and the organic layers combined. The resulting mixture was washed with ix200 mL of hydrogen chloride and ix300 mL of sodium bicarbonate. The resulting mixture was washed with ix300 mL of sodium chloride. The mixture was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (52:48). This resulted in8.52 g (75%) of A2, 2-((85,95, i0R, i iS,i35,i45,i75)-i i-hydroxy- i0,i3-dimethyl-3-oxo-2,3,6,7,8,9,i0,i i,i2,i3,i4,i5,i6,i7-tetradecahydro-iH-cyclopenta[a]phenanthren-i7-yl)-2- oxoethyl acetate as yellow oil.
References:
SAGE THERAPEUTICS, INC.;SALITURO, Francesco, Gerald;ROBICHAUD, Albert, Jean WO2013/188792, 2013, A2 Location in patent:Page/Page column 87; 88
50-23-7
768 suppliers
$21.00/1g

1173-26-8
71 suppliers
$45.00/25mg
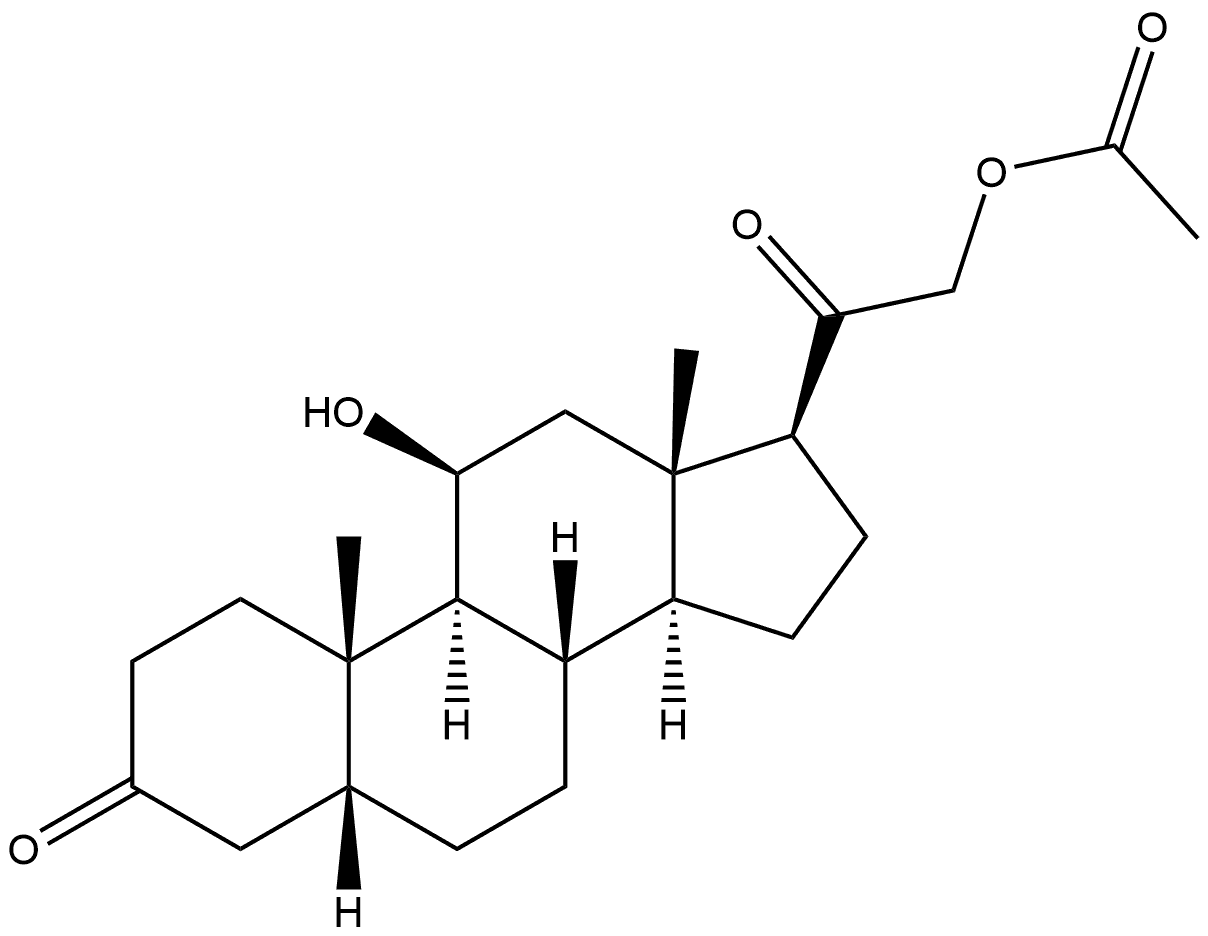
115073-83-1
1 suppliers
inquiry

1173-26-8
71 suppliers
$45.00/25mg
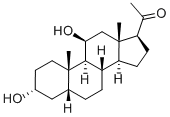
565-92-4
4 suppliers
inquiry

1173-26-8
71 suppliers
$45.00/25mg
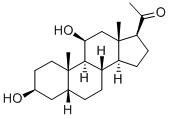
48200-75-5
3 suppliers
inquiry

1173-26-8
71 suppliers
$45.00/25mg
