
1,7-Octadiene synthesis
- Product Name:1,7-Octadiene
- CAS Number:3710-30-3
- Molecular formula:C8H14
- Molecular Weight:110.2
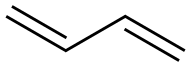
106-99-0
260 suppliers
$77.00/100mL

3710-30-3
114 suppliers
$14.00/1mL
Yield:3710-30-3 97.4 %Chromat.
Reaction Conditions:
Stage #1:buta-1,3-diene with 4-tert-Butylcatechol;sodium methylate;palladium diacetate in 1-methyl-pyrrolidin-2-one at -5 - 75; under 8250.83 Torr;Inert atmosphere;
Stage #2: with formic acid in 1-methyl-pyrrolidin-2-one under 15001.5 Torr;Product distribution / selectivity;Inert atmosphere;
Steps:
2
EXAMPLE 2100 ppm of Pd, 1,3-butadiene stabilized with 4-tert-butylcatecholUnder protective gas, 0.112 g of palladium acetate (47.5% Pd, from Acros), 0.312 g of 1,3-bis(2,4,6-tri-methylphenyl)imidazolium o-cresoxide-o-cresol and 0.108 g of sodium methoxide (>97%, from Merck) are dissolved in 200 g of freshly distilled N-methyl-pyrrolidone (NMP, 99.5%, from Merck) and stirred at 50° C. for 1 hour. This mixture is sucked into an evacuated 2 l autoclave from Büchi and then 240 g of 1,3-butadiene (1,3-butadiene 2.6, stabilized with approx. 100 ppm of 4-tert-butylcatechol (TBC), from Oxeno Olefinchemie GmbH) are condensed in at -5° C. The amount is measured by means of the weighing of the pressurized gas bottle. Thereafter, a pressure of 11 bar is applied by means of argon and the reactor is heated to 75° C. Within 30 minutes, 101 g of formic acid (98-100%, from Riedel-de Haen) are metered in. The pressure is kept constant at 20 bar by means of a valve. An amount of offgas of 31.7 l is measured. The continued reaction time is 15 min. After the cooling and decompression of the autoclave, the product mixture is analysed by GC. After a one-stage distillation at 50 mbar and a maximum bottom temperature of 80° C., a further analysis is effected by means of GC. Table 1 shows the particular analysis results in GC area %. TABLE 1 1,3-Butadiene 1,7-Octadiene 1,6-Octadiene 4-Vinylcyclohexene N-Methylpyrrolidone Further compounds Reactor 2.1 69.4 1.6 0.1 26.7 0.1 effluent Distillate 0 97.4 2.4 0.1 0 0.1 Distillation 0 6.2 0.3 0 92.7 0.8 bottoms The distillate obtained is 215.7 g of octadienes, which corresponds to an isolated yield of 89.3%. The purity based on 1,7-octadiene is 97.4%. The conversion of the formic acid is complete.
References:
EVONIK DEGUSSA GMBH US2009/287032, 2009, A1 Location in patent:Page/Page column 6
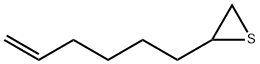
14122-59-9
0 suppliers
inquiry

3710-30-3
114 suppliers
$14.00/1mL
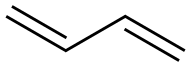
106-99-0
260 suppliers
$77.00/100mL
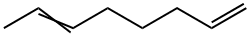
3710-41-6
5 suppliers
inquiry

3710-30-3
114 suppliers
$14.00/1mL
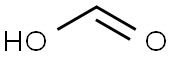
64-18-6
1112 suppliers
$22.12/250ML
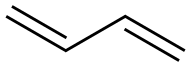
106-99-0
260 suppliers
$77.00/100mL
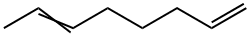
3710-41-6
5 suppliers
inquiry

3710-30-3
114 suppliers
$14.00/1mL

74-85-1
110 suppliers
$79.00/11l
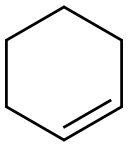
110-83-8
317 suppliers
$11.00/25g

3710-30-3
114 suppliers
$14.00/1mL