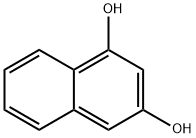
1,3-DIHYDROXYNAPHTHALENE synthesis
- Product Name:1,3-DIHYDROXYNAPHTHALENE
- CAS Number:132-86-5
- Molecular formula:C10H8O2
- Molecular Weight:160.17
1,3-Naphthalenediol is a derivative of naphthalene (N345600) and may have antibacterial activity against oral bacteria. It is obtained by decarboxylation of 1,3-dihydroxy-2-naphthoic acid.
135-19-3
744 suppliers
$9.00/1g

132-86-5
290 suppliers
$17.00/1g
Yield:-
Reaction Conditions:
with CYP175A1;dihydrogen peroxide in aq. phosphate buffer; pH=7.5 at 50; for 1 h;Kinetics;Enzymatic reaction;
Steps:
Enzymatic oxygenation of the substituted naphthalenes
General procedure: Oxygenation/oxidation of substituted naphthalene (see Table 1) by CYP175A1 was carried out by incubating 500 μL reaction mixture containing 10 μM CYP175A1, 500 μM substituted naphthalene (substrate) and 10 mM H2O2 in 50 mM KPi buffer (pH = 7.5) at 50 °C unless otherwise mentioned. The controls forthe reactions were carried out that contained all the reactants except CYP175A1 or except H2O2 in the assay mixture. Each reaction was allowed to proceed for 60 min unless otherwise stated.The reaction mixture was then extracted with 500 μL chloroform (containing 10% methanol) and the chloroform layer was collected,dried over anhydrous sodium sulfate, filtered through 0.2 μm syringe filter. The extracted reaction mixtures were then analyzed bydifferent mass spectrometry techniques.
References:
Banerjee, Shibdas;Goyal, Sandeep;Mazumdar, Shyamalava [Bioorganic Chemistry,2015,vol. 62,p. 94 - 105]
91-20-3
510 suppliers
$13.19/250mg

132-86-5
290 suppliers
$17.00/1g
569-42-6
142 suppliers
$10.00/1g
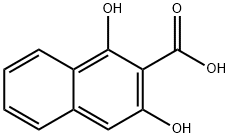
3147-58-8
65 suppliers
$47.00/100mg

132-86-5
290 suppliers
$17.00/1g
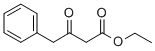
718-08-1
178 suppliers
$100.83/1g

132-86-5
290 suppliers
$17.00/1g
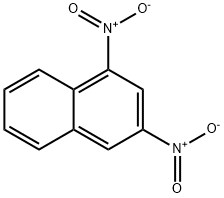
606-37-1
61 suppliers
$60.00/250 mg

132-86-5
290 suppliers
$17.00/1g