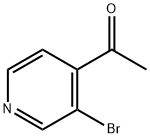
1-(3-Bromopyridin-4-yl)ethanone synthesis
- Product Name:1-(3-Bromopyridin-4-yl)ethanone
- CAS Number:111043-06-2
- Molecular formula:C7H6BrNO
- Molecular Weight:200.03
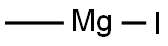
917-64-6
148 suppliers
$45.00/10ml
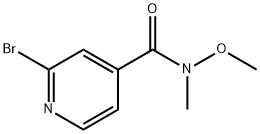
656257-69-1
27 suppliers
$51.00/100mg

111043-06-2
60 suppliers
$65.00/100mg
Yield:111043-06-2 100%
Reaction Conditions:
Stage #1: methyl magnesium iodide;2-bromo-N-methyl-N-(methyloxy)-4-pyridinecarboxamide in tetrahydrofuran;diethyl ether at 0 - 5; for 2 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;diethyl ether;
Steps:
22
Example 22; SynY-thesis of 2-bromo-4-acetylpyridine; 3 [0121] 2-Bromo-4- (N-methyl-N-methoxycarboxamide) pyridine (2. 4 g, 0.10 moles) was dissolved in anhydrous tetrahydrofuran (30 ml) and cooled to 0-5'C. After ten minutes, methylmagnesium iodide (3.0 M in ethyl ether) (1.8 ml) was added dropwise. After two hours at 0-5 °C, the reaction was quenched with saturated ammonium chloride. Extracted with ethyl acetate (2 x70 ml), washed with brine, dried over magnesium sulfate, filtered and stripped to give 2. 0g (100 % yield) of 2-bromo-4-acetylpyridine. lH NM : R (CDC13) 2.6 (3H, s), 7.6 (1H, d), 7.8 (1H, s), 8.5 (1H, d).
References:
WO2005/84439,2005,A1 Location in patent:Page/Page column 38

656257-69-1
27 suppliers
$51.00/100mg
75-16-1
270 suppliers
$12.00/10ml

111043-06-2
60 suppliers
$65.00/100mg

656257-69-1
27 suppliers
$51.00/100mg

111043-06-2
60 suppliers
$65.00/100mg
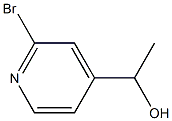
1220126-97-5
14 suppliers
inquiry

111043-06-2
60 suppliers
$65.00/100mg
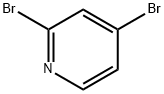
58530-53-3
303 suppliers
$4.00/1g
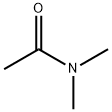
127-19-5
839 suppliers
$5.00/25g

111043-06-2
60 suppliers
$65.00/100mg