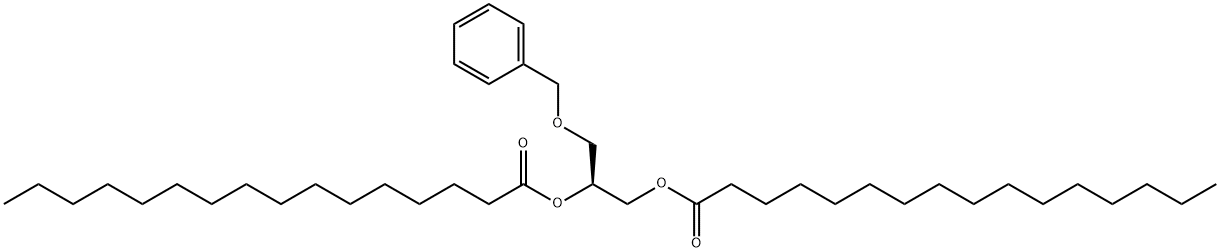
1,2-di-O-hexacecanoyl-3-O-benzyl-sn-glycerol synthesis
- Product Name:1,2-di-O-hexacecanoyl-3-O-benzyl-sn-glycerol
- CAS Number:30403-51-1
- Molecular formula:C42H74O5
- Molecular Weight:659.03
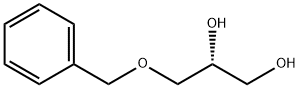
56552-80-8
128 suppliers
$16.00/100mg
112-67-4
273 suppliers
$10.00/5mL

30403-51-1
1 suppliers
inquiry
Yield:30403-51-1 378 mg
Reaction Conditions:
with pyridine;dmap in dichloromethane at 20;Inert atmosphere;
Steps:
3-O-Benzyl-1,2-di-O-hexadecanoyl-sn-glycerol (2)
To a stirred solution of(S)-glycidol (5 g, 67.5 mmol) and benzyl chloride (13 g) in DMF (40 mL) cooled at0 C was added a powder of NaH (2.4 g) slowly for 10 minutes. The mixture wasstirred at 0 C for 1 h and then at room temperature for 3 h. After the reactionvessel was cooled to 0 C, the mixture was treated carefully with droplets ofmethanol (2 mL) and stirred for 2 h at room temperature. The mixture was pouredinto aqueous sat. NaCl solution (100 mL) and extracted three times with a mixtureof ethyl acetate and toluene (1:10, 60 mL). The extracts were combined, washedwith NaCl aq. solution three times, dried over MgSO4, and evaporated underdiminished pressure to give I [(R)-3-O-benzyl-glycidol, CAS:14618-80-5] as a crudesyrup (11 g), which was used in the following reaction without purification. The crude syrup (5 g) was dissolved in acetic anhydride (30 mL), stirred for 30 minat 0 C, and then treated with CF3COOH (1.5 mL) under stirring. The solution wasstirred at 40 C for 6 h. The mixture was mixed with ethanol (5 mL), stirred for 4 h,and evaporated under diminished pressure together with a mixture of ethanol andtoluene to afford 1,2-diacetyl-sn-glycerol 3-benzyl ether II as syrup (8.2 g).The syrup of II (5 g) was dissolved in dry methanol (50 mL), treated with anhydrousK2CO3 (50 mg), and stirred at room temperature for 6 h. The reaction solution wasneutralized with Amberlyst-R-15 (H+) (Aldrich), filtered, and evaporated to give IIIas syrup (3.4 g). A part of the syrup (0.5 g) was purified by column chromatographyover SiO2 with a mixture of CHCl3 and methanol as eluents in a gradient mode from1000:1 to 10/1 (v/v) to give III (306 mg). The 1H NMR spectrum of III matched withthe reported data of (R)-(+)-3-benzyloxyl-1,2-propanediol (CAS: 13071-59-5)reported in literature [1-3]. 1H NMR (500 MHz, D2O); H 3.3~3.8 (4H, H1 + H3), 3.89(1H, m, H2), 4.52 (1H, d, -CH2Ph), 4.85 (1H, d, -CH2Ph), 7.36 (5H, m, -PhH5). The purified syrup of III (250 mg, 1.37 mmol) was dissolved in dry pyridine (15 mL)containing 4-(dimetylamino)pyridine (50 mg). To the stirred solution was added aCH2Cl2 solution (5 mL) of palmitoyl chloride (942 mg, 2.5 × 1.37 mmol), and the The purified syrup of III (250 mg, 1.37 mmol) was dissolved in dry pyridine (15 mL)containing 4-(dimetylamino)pyridine (50 mg). To the stirred solution was added aCH2Cl2 solution (5 mL) of palmitoyl chloride (942 mg, 2.5 × 1.37 mmol), and the purified product (378 mg) was identified as 3-O-benzyl-1,2-dipalmitoyl-sn-glycerol(2) (CAS: 30403-51-1) [4,5] by 1H NMR spectroscopy (Figures S1 and Figure S2) incomparison with 1H NMR data in a literature [5].
References:
Nishida, Yoshihiro;Yuan, Mengfei;Fukuda, Kazuo;Fujisawa, Kaito;Dohi, Hirofumi;Uzawa, Hirotaka [Beilstein Journal of Organic Chemistry,2017,vol. 13,p. 1999 - 2009] Location in patent:supporting information

56552-80-8
128 suppliers
$16.00/100mg
57-10-3
587 suppliers
$5.00/250mg

30403-51-1
1 suppliers
inquiry
16495-03-7
85 suppliers
$55.00/500mg

30403-51-1
1 suppliers
inquiry

56552-80-8
128 suppliers
$16.00/100mg

30403-51-1
1 suppliers
inquiry
![[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol](/CAS/GIF/1487-50-9.gif)