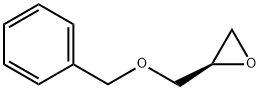
[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol synthesis
- Product Name:[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol
- CAS Number:1487-50-9
- Molecular formula:
- Molecular Weight:0
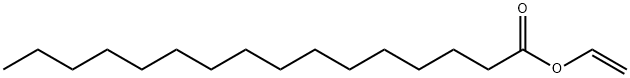
693-38-9
52 suppliers
$45.00/5mL
56552-80-8
121 suppliers
$16.00/100mg
![[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol](/CAS/GIF/1487-50-9.gif)
1487-50-9
0 suppliers
inquiry
Yield:1487-50-9 87%
Reaction Conditions:
with immobilised Candida antarctica lipase B in dichloromethane at 20; for 1.5 h;Enzymatic reaction;regioselective reaction;
Steps:
4.3. General Procedure A: enzymatic acylation procedure
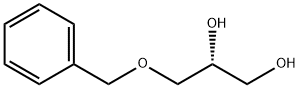
General procedure: To a mixture of benzyl protected glycerol (1.0 equiv.) and vinyl ester of the appropriate saturated fatty acid (1.05e1.3 equiv.) in CH2Cl2 (3.5 mL/mmol of substrate) was added immobilised Candida antarctica lipase B (CAL) (4e10 wt% of total combined mass of substrates). The resulting suspension was stirred at room temperature at a rate not to imperil the solid supported lipase. After stirring for 1.5 h (or until full consumption of starting material as indicated by TLC) the lipase was separated by filtration and the filtrate concentrated in vacuo to give the crude product which was purified by either flash column chromatography or recrystallisation (where appropriate). Note: 1-Acyl-3-O-alkylglycerols should be purified via column chromatography using 4% boric acid impregnated silica gel as they are prone to acyl migration. 4.4. 3-O-Benzyl-1-hexadecanoyl-sn-glycerol, (S)-13 (S)-13 was prepared using General Procedure A, with 3-Obenzyl-sn-glycerol (100 mg, 0.55 mmol, 1.0 equiv.), vinyl palmitate (117 mg, 0.63 mmol, 1.15 equiv.) and CAL (18 mg) in CH2Cl2 (2.0 mL), with a 1.5 h reaction time. Purification via column chromatography (4% boric acid impregnated silica gel, petroleum ether/EtOAc (40%)) gave (S)-13 (90 mg, 0.48 mmol, 87%) as a colourless oil; [a]D 25 1.11 (c 9.0, CH2Cl2); IR (NaCl, nmax/cm1 ) 3459, 3064, 3031, 2924, 2853, 1739, 1174; 1 H NMR (400 MHz, CDCl3) dH 7.39e7.27 (5H, m, ArH), 4.56 (2H, s, OCH2Ar), 4.19 (1H, dd, J 11.5 and 4.5 Hz, CH2OCO), 4.14 (1H, dd, J 11.5 and 6.0 Hz, CH2’OCO), 4.07e4.00 (1H, m, CHOCO), 3.56 (1H, dd, J 9.5 and 4.5 Hz, CH2OBn), 3.50 (1H, dd, J 9.5 and 6.0 Hz, CH2’OBn), 2.51 (1H, bs, OH), 2.32 (2H, t, J 7.5 Hz, CH2COO), 1.67e1.56 (2H, m, CH2CH2COO), 1.38e1.24 (24H, m, 12 CH2), 0.88 (3H, t, J 6.5 Hz, CH3); 13C NMR (100 MHz, CDCl3) dC 174.1, 137.8, 128.6, 128.0, 127.9, 73.7, 71.0, 69.1, 65.5 34.3, 32.1, 29.83, 29.80, 29.75, 29.6, 29.5, 29.4, 29.3, 25.1, 22.8, 14.3; HRMS (ESI) calc. for C26H44O4Na [MNa] 443.3132, found 443.3127.
References:
Gudmundsson, Haraldur G.;Linderborg, Kaisa M.;Kallio, Heikki;Yang, Baoru;Haraldsson, Gudmundur G. [Tetrahedron,2020,vol. 76,# 2,art. no. 130813]

56552-80-8
121 suppliers
$16.00/100mg
57-10-3
570 suppliers
$5.00/250mg
![[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol](/CAS/GIF/1487-50-9.gif)
1487-50-9
0 suppliers
inquiry
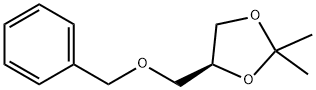
16495-03-7
85 suppliers
$55.00/500mg
![[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol](/CAS/GIF/1487-50-9.gif)
1487-50-9
0 suppliers
inquiry
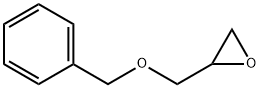
2930-05-4
120 suppliers
$8.00/5g
![[S,(+)]-3-O-Benzyl-1-O-palmitoyl-L-glycerol](/CAS/GIF/1487-50-9.gif)
1487-50-9
0 suppliers
inquiry