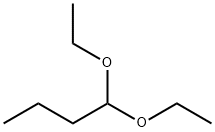
1,1-Diethoxybutane synthesis
- Product Name:1,1-Diethoxybutane
- CAS Number:3658-95-5
- Molecular formula:C8H18O2
- Molecular Weight:146.23
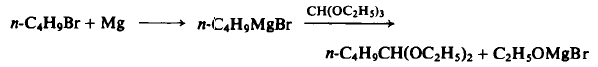

123-72-8
435 suppliers
$12.32/25ML
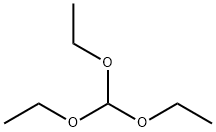
122-51-0
506 suppliers
$10.00/5ml

3658-95-5
53 suppliers
$25.00/5g
Yield:3658-95-5 94%
Reaction Conditions:
aminosulfonic acid at 25; for 4 h;
References:
Gong, Weizhong;Wang, Bo;Gu, Yanglong;Yan, Liang;Yang, Liming;Suo, Jishuan [Synthetic Communications,2004,vol. 34,# 23,p. 4243 - 4247]

64-17-5
757 suppliers
$10.00/50g

123-72-8
435 suppliers
$12.32/25ML

3658-95-5
53 suppliers
$25.00/5g
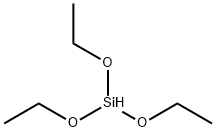
998-30-1
256 suppliers
$30.00/25 g

123-72-8
435 suppliers
$12.32/25ML

3658-95-5
53 suppliers
$25.00/5g
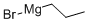
927-77-5
116 suppliers
$194.00/250g

122-51-0
506 suppliers
$10.00/5ml

3658-95-5
53 suppliers
$25.00/5g

64-17-5
757 suppliers
$10.00/50g
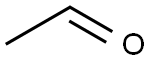
75-07-0
425 suppliers
$14.00/5mL
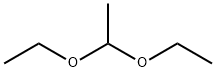
105-57-7
321 suppliers
$18.00/25mL
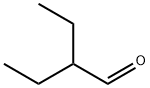
97-96-1
142 suppliers
$10.00/5mL

3658-95-5
53 suppliers
$25.00/5g