Magnesium hydride
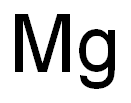
|
- $236.25 - $945
- Product name: Magnesium hydride
- CAS: 7693-27-8
- MF: H2Mg
- MW: 26.32
- EINECS:231-705-3
- MDL Number:MFCD00011101
- Synonyms:magnesium dihydride ;MAGNESIUM HYDRIDE, 90% (REMAINDER MAGNESIUM);Magnesium hydride hydrogen, storage grade;agnesiumhydride,98%;MAGNESIUM HYDRIDE;MAGNESIUMHYDRIDE-NI-DOPED;Dihydridemagnesium;Dihydrogen magnesium salt
2 prices
Selected condition:
Brand
- Iris Biotech GmbH
Package
- 10mg
- 50mg
- ManufacturerIris Biotech GmbH
- Product numberHAA3320
- Product descriptionMG-H2
- Packaging10mg
- Price$236.25
- Updated2021-12-16
- Buy
- ManufacturerIris Biotech GmbH
- Product numberHAA3320
- Product descriptionMG-H2
- Packaging50mg
- Price$945
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Iris Biotech GmbH | HAA3320 | MG-H2 | 10mg | $236.25 | 2021-12-16 | Buy |
| Iris Biotech GmbH | HAA3320 | MG-H2 | 50mg | $945 | 2021-12-16 | Buy |
Properties
Melting point :>250°C (dec.)
Density :1.45
storage temp. :water-free area
solubility :reacts with H2O
form :white tetragonal crystals
color :white tetragonal crystals, crystalline
Sensitive :Moisture Sensitive
EPA Substance Registry System :Magnesium hydride (MgH2) (7693-27-8)
Density :1.45
storage temp. :water-free area
solubility :reacts with H2O
form :white tetragonal crystals
color :white tetragonal crystals, crystalline
Sensitive :Moisture Sensitive
EPA Substance Registry System :Magnesium hydride (MgH2) (7693-27-8)
Safety Information
| Symbol(GHS): |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
MgH2 contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen-storage medium. It was discovered in 1912, during the pyrolysis of ethyl magnesium iodide (a Grignard reagent), which produced small amount of MgH2. In 1951, preparation from the elements was first reported involving direct hydrogenation of Mg metal at high pressure and temperature (200 atmospheres, 500°C)with magnesium iodide as a catalyst:Mg+H2→MgH2
Lower temperature production from Mg and H2 using nano-crystalline Mg produced in ball mills has been investigated. Other preparations include:
1. The hydrogenation of magnesium anthracene under mild conditions:
Mg(anthracene)+H2→MgH2+C14H10 2. The reaction of diethyl magnesium with LiAlH4
3. An adduct of complexed MgH2, e.g. MgH2·THF by the reaction of phenylsilane (C6H8Si) and dibutyl magnesium in ether or hydrocarbon solvents in the presence of THF (C4H8O) using TMEDA (Tetramethylethylenediamine=(CH3)2NCH2CH2N (CH3)2) as a ligand.
More related product prices
Benzylmagnesium chloride Ethylmagnesium chloride PHENYLMAGNESIUM CHLORIDE PHENYLMAGNESIUM BROMIDE Vinylmagnesium bromide METHYLMAGNESIUM BROMIDE ISOPROPYLMAGNESIUM CHLORIDE ETHYLMAGNESIUM BROMIDE Butylmagnesium chloride TERT-BUTYLMAGNESIUM CHLORIDE Methylmagnesium chlorideRelated product price
- 15366-08-2
$93-800 - Benzylmagnesium chloride
$75.65-1180.84 - Ethylmagnesium chloride
$45-499.88
Suppliers and manufacturers
Hangzhou FandaChem Co.,Ltd.
Hubei Jusheng Technology Co.,Ltd.
Hubei xin bonus chemical co. LTD
Antai Fine Chemical Technology Co.,Limited
ANHUI WITOP BIOTECH CO., LTD
PT CHEM GROUP LIMITED
LEAPCHEM CO., LTD.
Shanghai Likang New Materials Co., Limited
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
Mainchem Co., Ltd.





