- 3-Oxetanone
-

- $10.00 / 1KG
-
2025-04-23
- CAS:6704-31-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
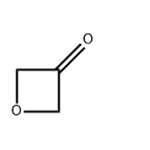
- 3-Oxetanone
-
- $0.00 / 1kg
-
2025-04-04
- CAS:6704-31-0
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
- 3-Oxetanone
-

- $0.00 / 25KG
-
2025-03-21
- CAS:6704-31-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
|
| | 3-Oxetanone Basic information |
| Product Name: | 3-Oxetanone | | Synonyms: | oxentan-3-on;3-Oxetanone;3-oxetanon;Oxetan-3-one 95%;3-Oxetanone, 98%+;EOS-60380;Oxetan-3-one;1,3-Epoxy-2-propanone | | CAS: | 6704-31-0 | | MF: | C3H4O2 | | MW: | 72.06 | | EINECS: | 664-396-2 | | Product Categories: | 1 | | Mol File: | 6704-31-0.mol | 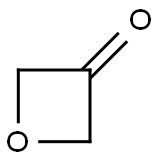 |
| | 3-Oxetanone Chemical Properties |
| Boiling point | 140 °C | | density | 1.231 | | Fp | 53 °C | | refractive index | 1.426 | | storage temp. | -20°C | | solubility | Miscible with tetrahydrofuran. | | form | clear liquid | | color | Colorless to Red to Green | | InChI | InChI=1S/C3H4O2/c4-3-1-5-2-3/h1-2H2 | | InChIKey | ROADCYAOHVSOLQ-UHFFFAOYSA-N | | SMILES | O1CC(=O)C1 |
| Hazard Codes | Xn | | Risk Statements | 10-22-37/38-41-43 | | Safety Statements | 16-26-36/37/39 | | RIDADR | 1993 | | WGK Germany | 3 | | HazardClass | 3 | | HazardClass | IRRITANT | | PackingGroup | Ⅲ | | HS Code | 29329990 |
| | 3-Oxetanone Usage And Synthesis |
| Chemical Properties | Colorless liquid | | Uses | 3-Oxetanone can be used to synthesize:
- Various oxetane-containing lead compounds with improved solubility, reduced lipophilicity and amphiphilicity.
- (Hydroxymethyl)oxazoles and (hydroxymethyl)thiazoles via single-step microwave mediated reaction with primary amides and thioamides, respectively.
- Oxetane containing spirocycles through thermal 1,3-dipolar cycloaddition reaction with α-amino acids or secondary α-amino acid esters.
| | Uses | 3-Oxetanone is used as a starting material for the preparation of other oxetanes compounds, which are of pharmacological interest. It is also considered as an object for theoretical studies. | | Definition |
3-oxetanone (1,3-epoxy2-propanone, c?C3H4O2) is a highly strained molecule functional in ring expansion and addition reactions. This molecule has also been proposed as a decomposition product of acetonylperoxy radical in the atmosphere. 3-oxetanone is suspected in the decomposition of the acetonylperoxy radical, an intermediate formed during the atmospheric processing of acetone. Atmospheric acetone degradation produces the acetonyl radical, CH3C(O)CH2, which reacts with O2 to form the acetonylperoxy radical. Rate constants predicted by a canonical variational theory/small-curvature tunneling method show that the radical isomerising produces 3-oxetanone and hydroxyl radical. These are the dominant products in the 250?2000 K range. There is no significant branching to other products below 1200 K. The only published study of the dissociation of 3-oxetanone is a Rice-Ramsperger-KasselMarcus (RRKM) analysis of the unimolecular rate constant for the photolysis of 3-oxetanone, producing ketene and formaldehyde[1-2].
| | General Description | Product may polymerize over time during storage. | | References |
[1] Sumitra Godara, Manikandan Paranjothy, Pooja Verma. “Dissociation Chemistry of 3-Oxetanone in the Gas Phase.” The Journal of Physical Chemistry A 121 36 (2017): 6679–6686.
[2] Emily M. Wright. “Pyrolysis Reactions of 3-Oxetanone.” The Journal of Physical Chemistry A 119 29 (2015): 7966–7972.
|
| | 3-Oxetanone Preparation Products And Raw materials |
|