- Laquinimod
-

- $37.00 / 10mg
-
2024-11-19
- CAS:248281-84-7
- Min. Order:
- Purity: 98.84%
- Supply Ability: 10g
|
| | CIVENTICHEM CV-4057 Basic information |
| Product Name: | CIVENTICHEM CV-4057 | | Synonyms: | LAQ;Laquinimod potassium salt;5-Chloro-N-Et-4-hydroxy-1-methyl-2-oxo-N-Ph-1,2-dihydroquinoline-3-carboxamide;Laquinimod, >=98%;CIVENTICHEM CV-4057;LAQUINIMOD,5-CHLORO-4-HYDROXY-1-METHYL-2-OXO-1,2-DIHYDRO-QUINOLINE-3-CARBOXYLIC ACID ETHYL-PHENYL-AMIDE;5-Chloro-4-hydroxy-1-Methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic acid ethyl-phenyl-aMide;LaquiniMod, SAIK-MS coMpound, ABR-215062 | | CAS: | 248281-84-7 | | MF: | C19H17ClN2O3 | | MW: | 356.8 | | EINECS: | 692-155-1 | | Product Categories: | Inhibitors;api;Heterocycles | | Mol File: | 248281-84-7.mol | 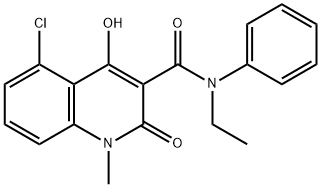 |
| | CIVENTICHEM CV-4057 Chemical Properties |
| Melting point | 201 °C (decomp) | | Boiling point | 484.8±45.0 °C(Predicted) | | density | 1.395±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Soluble in DMSO (>25 mg/ml) | | form | solid | | pka | 4.50±1.00(Predicted) | | color | Off-white | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 2811 6.1 / PGIII |
| | CIVENTICHEM CV-4057 Usage And Synthesis |
| Description | Laquinimod (248281-84-7) is an immunomodulator1 that inhibits inflammation2 and autoimmunity3,4 via activation of the aryl hydrocarbon receptor (AhR)5. It has been investigated as a treatment for multiple sclerosis6 and has shown efficacy in Huntington’s disease models7,8. | | Definition | ChEBI: Laquinimod is an aromatic amide. | | in vitro | abr-215062 was shown to completely inhibit the development of murine acute experimental autoimmune encephalomyelitis (eae) [1]. | | in vivo | abr-215062 dose-dependently inhibited disease and showed better disease inhibitory effects as compared to roquinimex (linomide). furthermore, abr-215062 inhibited the inflammation of both cd4+ t cells and macrophages into central nervous tissues [2]. | | References | J?nsson et al. (2004), Synthesis and Biological Evaluation of New 1,2-Dihydro-4-hydroxy-2-oxo-3-quinolinecarboxamides for Treatment of Autoimmune Disorders: Structure-Activity Relationship; Med. Chem. 47 2075
Rothhammer et al. (2021); Aryl Hydrocarbon Receptor Activation in Astrocytes by Laquinimod Ameliorates Autoimmune Inflammation in the CNS, Neurol. Neuroimmunol. Neuroinflamm., 8 e946
Pitarokoili et al. (2014), Laquinimod exerts strong clinical and immunomodulatory effects in Lewis rat experimental autoimmune neuritis; Neuroimmunol., 274 38
Ott et al. (2019), Laquinimod, a prototypic quinoline-3-carboxamide and aryl hydrocarbon receptor agonist, utilizes CD155-mediated natural killer/dendritic cell interaction to suppress CNS autoimmunity; Neuroinflammation, 16 49
Kaye et al. (2016), Laquinimod arrests experimental autoimmune encephalitis by activating the aryl hydrocarbon receptor; Natl. Acad. Sci. USA., 113 E6145
Th?ne and Linker (2016), Laquinimod in the treatment of multiple sclerosis: a review of the data so far; Drug Des. Devel. Ther., 10 1111
Dobson et al. (2016), Laquinimod dampens hyperactive cytokine production in Huntington’s disease patient myeloid cells; Neurochem., 137 782
Garcia-Miralles et al. (2016), Laquinimod rescues striatal, cortical and white matter pathology and results in modest behavioural improvements in the YAC128 model of Huntington disease; Rep., 6 31652 |
| | CIVENTICHEM CV-4057 Preparation Products And Raw materials |
|