|
|
| Product Name: | 4,4'-Oxybis(benzoyl Chloride) | | Synonyms: | 4-(4-carbonochloridoylphenoxy)benzoyl chloride;4,4-Dicarboxylic acid diphenyl oxide dichloroanhydride;p,p'-Oxybis(benzoyl chloride);4,4'-OXYBISBENZOYL CHLORIDE;4,4'-Oxybisbenzoyl chloride(DEDC/ODBC);4-(Chlorocarbonyl)phenyl ether;Diphenyl ether 4,4'-dicarbonyl dichloride;Diphenyl oxide 4,4'-dicarbonyl chloride | | CAS: | 7158-32-9 | | MF: | C14H8Cl2O3 | | MW: | 295.12 | | EINECS: | 808-159-5 | | Product Categories: | 7158-32-9 | | Mol File: | 7158-32-9.mol | 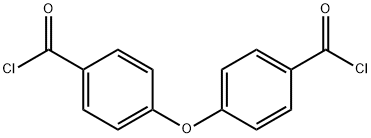 |
| | 4,4'-Oxybis(benzoyl Chloride) Chemical Properties |
| Melting point | 22-23℃ | | Boiling point | 404℃ | | density | 1.386 | | Fp | 164℃ | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | solubility | soluble in Toluene | | form | powder to crystaline | | color | White to Almost white | | InChI | InChI=1S/C14H8Cl2O3/c15-13(17)9-1-5-11(6-2-9)19-12-7-3-10(4-8-12)14(16)18/h1-8H | | InChIKey | OSUWBBMPVXVSOA-UHFFFAOYSA-N | | SMILES | O(C1=CC=C(C=C1)C(Cl)=O)C1=CC=C(C=C1)C(Cl)=O |
| RIDADR | UN 3261 8/PG II | | RTECS | DM6664000 | | HS Code | 2918.99.4700 | | HazardClass | 8 | | PackingGroup | II |
| | 4,4'-Oxybis(benzoyl Chloride) Usage And Synthesis |
| Physical Form | Crystal - Powder | | Uses | 4,4'-Oxybis(benzoyl Chloride) is a monomer used in high-performance polymers for the preparation of a new low-temperature curing photosensitive polymer (PHA-6F). The polymer exhibits high transparency at 365 nm (i-line).
The photosensitivity and contrast of the 2.4 μm thick film were 15 mJ/cm2 and 2.5, respectively. | | Uses | 4,4'-OXYBISBENZOYL CHLORIDE is a useful research chemical. | | Synthesis | 4,4′-oxybisbenzaldehyde is reacted with chlorine to produce 4,4'-Oxybis(benzoyl Chloride) in high yield without using a large amount of reaction solvent. A 300 ml four-necked flask equipped with a stirrer, a thermometer and a reflux condenser was charged with 90.6 g (0.40 mol) of 4,4′-oxybisbenzaldehyde and 158.6 g of PCBTF, and heated and stirred to 143 ° C. The temperature was raised to. A high pressure mercury lamp was turned on and irradiated with light. The reaction was started by supplying chlorine gas at a chlorine gas blowing rate of 0.06 mol / hour so as to maintain a reaction temperature of 143 ° C. (reflux). After 15 hours from the start of the reaction, disappearance of the raw materials was confirmed by gas chromatography, and the reaction was completed. When this was analyzed using gas chromatography, the reaction yield of 4,4'-Oxybis(benzoyl Chloride) was 98.6% by mass. |
| | 4,4'-Oxybis(benzoyl Chloride) Preparation Products And Raw materials |
|