- THIOMERSAL
-

- $23.00 / 1kg
-
2024-01-08
- CAS:54-64-8
- Min. Order: 1kg
- Purity: 99.96%
- Supply Ability: 50kg
- Thimerosal
-

- $25.00 / 1KG
-
2021-11-15
- CAS:54-64-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5ton/Month
- Thimerosal
-

- $0.00 / 1KG
-
2020-05-04
- CAS:54-64-8
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 600 Tons
|
| | Thimerosal Basic information |
| | Thimerosal Chemical Properties |
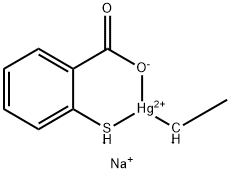
| Melting point | 234-237 °C (dec.)(lit.) | | Fp | 250 °C | | storage temp. | Store at RT. | | solubility | methanol: 0.1 g/mL, ≤ 10 TE CF | | form | Powder | | color | white to off-white | | PH | 6.0~8.0 (10g/L, 25℃) | | Water Solubility | 1 G/ML (20 ºC) | | Merck | 13,9389 | | BRN | 8169555 | | Stability: | Stable. May degrade in sunlight. Incompatible with strong acids, strong bases, strong oxidizing agents, iodine, heavy metal salts. | | InChI | InChI=1S/C7H6O2S.C2H4.Hg.Na/c8-7(9)5-3-1-2-4-6(5)10;1-2;;/h1-4,10H,(H,8,9);1H,2H3;;/q;-1;2*+1/p-1 | | InChIKey | RTKIYNMVFMVABJ-UHFFFAOYSA-L | | SMILES | O=C1[O-][Hg+2]([CH-]C)[SH-]C2=CC=CC=C12.[Na+] | | CAS DataBase Reference | 54-64-8(CAS DataBase Reference) | | EPA Substance Registry System | Thimerosal (54-64-8) |
| | Thimerosal Usage And Synthesis |
| Description | Since the 1930s, thimerosal (sodium ethylmercury thiosalicylate)
has been used in the manufacture of vaccines to
prevent fungal or bacterial contamination of multidose preparations.
The single-dose vaccines are much more expensive and
thus the use of the preservative significantly reduces the cost of
vaccination. There is controversy as to whether the use of
thimerosal in vaccines is an etiological factor in autism and other
neurodevelopmental disorders such as attention deficit hyperactivity
disorder. It has been estimated that children exposed to
mercury-containing vaccines could be exposed to levels of
mercury far beyond those considered safe by the US Food and
DrugAdministration (FDA) standards. Therefore, in 1999, theUS
Public Health Service agencies urged vaccine manufacturers to
stop or limit the use of the preservative in vaccine formulations.
Today, the vaccines on the US Recommended Childhood and
Adolescent Immunization Schedule are thimerosal-free although
some formulations of the influenza vaccine still contain the
mercury-containing preservative. The Centers for DiseaseControl
and Prevention (CDC) has discounted an association between
exposure to this organic ethylmercury compound and autism.
This is based on studies showing that the risk of autism is not
increased in children treated with vaccines containing the
preservative compared with children who were vaccinated with
thimerosal-free formulations. Furthermore, in Denmark, the use
of the preservative was discontinued after 1992. After removal
of thimerosal, there was a continued rise in new cases of autism. | | Chemical Properties | Slightly beige powder | | Chemical Properties | Thimerosal is a light cream-colored crystalline powder with a slight,
characteristic odor. | | Originator | Timeolate,Lifar | | Uses | nootropic Anti-Alzheimer’s drug main ingredient of Neupramir derived from Piracetam but 8-30 times more potent | | Uses | Thiomersal is an organomercury compound that is used as an antiseptic and antifungal agent It is used as a vaccine preservative. During cell culture, thiomersal is used to prevent overgrowth in fibroblast cells 1. It has been used to preserve Atovaquone 2. | | Uses | antifungal, antimicrobial | | Uses | Thimerosal is used as ophthalmie preservative; topieal anti-infective; topical veterinary antibacterial and antifungal agent; bacteriostat; fungistat;
preservative in vaccines, antitoxins, skin-testing allergens, antiseptics, contact-Iens solutions, and cosmetic products like eye makeup. | | Uses | Pharmaceutic aid (preservative). | | Definition | ChEBI: An alkylmercury compound (approximately 49% mercury by weight) used as an antiseptic and antifungal agent. | | Production Methods | Thimerosal is prepared by the interaction of ethylmercuric chloride,
or hydroxide, with thiosalicylic acid and sodium hydroxide, in
ethanol (95%). | | Manufacturing Process | To a solution or suspension in alcohol of 0.1 mole of methyl mercuric chloride
is added 0.1 mole of sodium hydroxide in water and 0.1 mole of thiosalicylic
acid in ethanol. The product is poured into water, whereupon; the methyl
mercurithiosalicylic acid is precipitated, since it is insoluble in water. This
precipitate can be collected on a filter, and washed well with water to remove
all the alcohol, salts, and free inorganic acids. The washed precipitate may
then be dissolved in a water solution of sodium hydroxide, or, better, in a
water solution of sodium bicarbonate. This produces the water-soluble salt of
the methyl mercurithiosalicylic acid.The methyl mercurithiosalicylic acid is a white solid which melts at about
171°C. It is soluble in alcohol and in ether. It is soluble in either sodium
bicarbonate or sodium hydroxide solution, to form the corresponding salt;
which is suitable for intravenous injection.
The alkali metal salts, such as the sodium and potassium salts, of this acid,
are readily a soluble in water; so are its ammonium salts, and many (probably
all) of its alkyl-ammonium salts; but the alkaline earth salts, such as the
calcium salt, are insoluble in water. | | Brand name | Thiomersal is INN and BAN;Thiobactal. | | Therapeutic Function | Antiseptic, Pharmaceutic aid | | General Description | Light cream-colored crystalline powder with a slight odor: pH (1% aqueous solution) 6.7. Slight odor. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Thimerosal is incompatible with strong oxidizing agents and strong bases. The oxidation of Thimerosal is greatly accelerated by traces of copper ions. Incompatible with acids, iodine, heavy metal salts and many alkaloids. Can be absorbed by rubber caps. . Stable in air, but not sunlight. May discolor on exposure to light. Dilute aqueous solutions are fairly stable to heat but sensitive to light. Solutions are less stable to heat when acidic than when alkaline. Solutions are most stable to light at pH 5 to 7. Solutions are unstable to heat but not to light in the presence of copper, iron or zinc ions but not in the presence of calcium or magnesium ions. | | Hazard | Toxic by ingestion, inhalation. | | Fire Hazard | Flash point data for Thimerosal are not available; however, Thimerosal is probably combustible. | | Pharmaceutical Applications | Thimerosal has been used as an antimicrobial preservative in
biological and pharmaceutical preparations since the 1930s.
It is used as an alternative to benzalkonium chloride and other
phenylmercuric preservatives, and has both bacteriostatic and
fungistatic activity. Increasing concerns over its safety have,
however, led to questions regarding its continued use in formulations.
Thimerosal is also used in cosmetics and to
preserve soft contact lens solutions.
Therapeutically, thimerosal is occasionally used as a bacteriostatic
and fungistatic mercurial antiseptic, which is usually applied
topically at a concentration of 0.1% w/w. However, its use is
declining owing to its toxicity and effects on the environment. | | Contact allergens | Thiomersal is an organic mercury salt prepared by react-
ing ethylmercuric chloride (or ethylmercuric hydroxide)
with thiosalicylic acid. It is still used as a disinfectant
and a preservative agent, but less commonly than previ-
ously, especially in contact lens fluids, eyedrops, and
vaccines. The ethylmercuric moiety is the major aller-
genic determinant, sometimes associated with mercury
sensitivity. Thiomersal is an indicator of photosensitiv-
ity to piroxicam, through its thiosalicylic moiety. | | Safety Profile | Poison by ingestion,
subcutaneous, and intravenous routes.
Experimental teratogenic and reproductive
effects. An eye irritant. Questionable carcinogen with experimental neoplastigenic
data. Mutation data reported. An ophthalmic
preservative, a topical anti-infective, topical
veterinary antibacterial and antifungal agent.
An FDA over-the-counter drug. When
heated to decomposition it emits very toxic
fumes of Hg, Na2O, and SOx. See also
MERCURY COMPOUNDS. | | Safety | Thimerosal is widely used as an antimicrobial preservative in
parenteral and topical pharmaceutical formulations. However,
concern over the use of thimerosal in pharmaceuticals has increased
as a result of a greater awareness of the toxicity of mercury and
other associated mercury compounds. The increasing number
of reports of adverse reactions, particularly hypersensitivity,
to thimerosal and doubts as to its effectiveness as a preservative
have led to suggestions that it should not be used as a preservative in
eye drops or vaccines. In both Europe and the USA,
regulatory bodies have recommended that thimerosal in vaccines be
phased out.
More recent studies assessing the safety of thimerosal in vaccines
have, however, suggested that while the risk of hypersensitivity
reactions is present, the relative risk of neurological harm in infants
is negligible given the quantities of thimerosal present in
vaccines. Regulatory bodies in Europe and the USA have
therefore updated their advice on the use of thimerosal in vaccines
by stating that while it would be desirable for thimerosal not to be
included in vaccines and other formulations the benefits of vaccines
far outweigh any risks of adverse effects associated with their
use.
The most frequently reported adverse reaction to thimerosal,
particularly in vaccines, is hypersensitivity, usually with
erythema and papular or vesicular eruptions. Although not all
thimerosal-sensitive patients develop adverse reactions to vaccines
containing thimerosal, there is potential risk. Patch testing in
humans and animal experiments have suggested that 0.1% w/v
thimerosal can sensitize children. The incidence of sensitivity to
thimerosal appears to be increasing; a study of 256 healthy subjects
showed approximately 6% with positive sensitivity.
Adverse reactions to thimerosal used to preserve contact lens
solutions have also been reported. Reactions include ocular redness,
irritation, reduced lens tolerance, and conjunctivitis. One
estimate suggests that approximately 10% of contact lens wearers
may be sensitive to thimerosal.
Thimerosal has also been associated with false positive reactions
to old tuberculin, ototoxicity, and an unusual reaction to
aluminum in which a patient suffered a burn 5 cm in diameter at
the site of an aluminum foil diathermy electrode after preoperative
preparation of the skin with a 0.1% w/v thimerosal solution in
ethanol (50%). Investigation showed that considerable heat was
generated when such a solution came into contact with aluminum.
An interaction between orally administered tetracyclines and
thimerosal, which resulted in varying extents of ocular irritation,
has been reported in patients using a contact lens solution preserved
with thimerosal.
Controversially, some have claimed a connection between the
use of thimerosal in vaccines and the apparent rise in the incidence
of autism. However, recent studies have shown no association
between thimerosal exposure and autism.
Serious adverse effects and some fatalities have been reported
following the parenteral and topical use of products containing
thimerosal. Five fatal poisonings resulted from the use of 1000 times
the normal concentration of thimerosal in a chloramphenicol
preparation for intramuscular injection.
Ten out of 13 children died as a result of treatment of umbilical
hernia (omphaloceles) with a topical tincture of thimerosal. It
has therefore been recommended that organic mercurial disinfectants
should be restricted or withdrawn from use in hospital since
absorption occurs readily through intact membranes.
In a case of attempted suicide, a 44-year-old man drank
83 mg/kg of a thimerosal-containing solution. Despite spontaneously vomiting after 15 minutes, gastric lavage and administration
of chelating agents on hospital admission, serious symptoms
ultimately ending in coma occurred. The patient survived and after
5 months treatment made a full recovery except for sensory defects
in two toes.
LD50 (mouse, oral): 91 mg/kg
LD50 (rat, oral): 75 mg/kg
LD50 (rat, SC): 98 mg/kg | | Environmental Fate | Thimerosal is a cream-colored crystalline powder; it is water
soluble and thus the compound can leach into groundwater.
Aquatic toxicity has been demonstrated at relatively high
concentrations. | | storage | Thimerosal is stable at normal temperatures and pressures;
exposure to light may cause discoloration.
Aqueous solutions may be sterilized by autoclaving but are
sensitive to light. The rate of oxidation in solutions is increased by
the presence of trace amounts of copper and other metals. Edetic
acid or edetates may be used to stabilize solutions but have been
reported to reduce the antimicrobial efficacy of thimerosal
solutions.
The solid material should be stored in a well-closed container,
protected from light, in a cool, dry place. | | Purification Methods | Recrystallise this antibacterial from EtOH/Et2O. HIGHLY TOXIC. [Trikojus Nature 158 472 1940, Beilstein 10 III 213.] | | Toxicity evaluation | Not much is known about the toxic effects of ethylmercury and
most toxicologists have assumed that the toxicity is similar to
that caused by MeHg. However, because of the differences in
the toxicokinetic profiles of the organic mercury salts (see
above), this may not be a valid comparison. Methylmercury is
a known neurotoxin. However, the neurotoxicity is very
complex and depends on the duration of exposure, dose, and
the age of the individual. Mercury salts have a strong affinity for
thiol groups and this is likely to play a role in mediating their
neurotoxicity. Glutathione depletion has been observed after
exposure to ethylmercury. Some in vitro studies indicate that
oxidative stress leading to lipid peroxidation and DNA damage
may play a role in the mechanism of toxicity. Organic mercury
salts may exert their toxicity by multiple diverse pathways that
have yet to be precisely defined. | | Incompatibilities | Incompatible with aluminum and other metals, strong oxidizing
agents, strong acids and bases, sodium chloride solutions,
lecithin, phenylmercuric compounds, quaternary ammonium compounds,
thioglycolate, and proteins. The presence of sodium
metabisulfite, edetic acid, and edetates in solutions can reduce the
preservative efficacy of thimerosal.
In solution, thimerosal may be adsorbed by plastic packaging
materials, particularly polyethylene. It is strongly adsorbed by
treated or untreated rubber caps that are in contact with
solutions.
When it was used with cyclodextrin, the effectiveness of
thimerosal was reduced; however, this was related to the lipid
nature of the other ingredients in the preparation. | | Regulatory Status | Included in the FDA Inactive Ingredients Database (IM, IV, and SC
injections; ophthalmic, otic, and topical preparations). Included in
nonparenteral and parenteral medicines licensed in the UK. In the
UK, the use of thimerosal in cosmetics is limited to 0.003% w/w
(calculated as mercury) as a preservative in shampoos and haircreams,
which contain nonionic emulsifiers that would render other
preservatives ineffective. The total permitted concentration (calculated
as mercury) when mixed with other mercury compounds is
0.007% w/w. Included in the Canadian List of Acceptable Nonmedicinal
Ingredients. |
| | Thimerosal Preparation Products And Raw materials |
|