- Tubacin
-

- $77.00 / 1mg
-
2025-04-28
- CAS:537049-40-4
- Min. Order:
- Purity: 95.63%
- Supply Ability: 10g
|
| | Tubacin Basic information | | Found |
| Product Name: | Tubacin | | Synonyms: | Tubacin;N1-(4-((2R,4R,6S)-4-((4,5-diphenyloxazol-2-ylthio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-dioxan-2-yl)phenyl)-N8-hydroxyoctanediamide;N1-[4-[(2R,4R,6S)-4-[[(4,5-diphenyl-2-oxazolyl)thio]methyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]phenyl]-N8-hydroxy-octanediamide Tubacin;N1-[4-[(2R,4R,6S)-4-[[(4,5-diphenyl-2-oxazolyl)thio]methyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]phenyl]-N8-hydroxy-octanediamide;Tubacin, >=98%;N1-(4-((2R,4R,6S)-4-(((4,5-Diphenyloxazol-2-yl)thio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-di;BML-GR362;Tubacin (BML-GR362) | | CAS: | 537049-40-4 | | MF: | C41H43N3O7S | | MW: | 721.86 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 537049-40-4.mol | 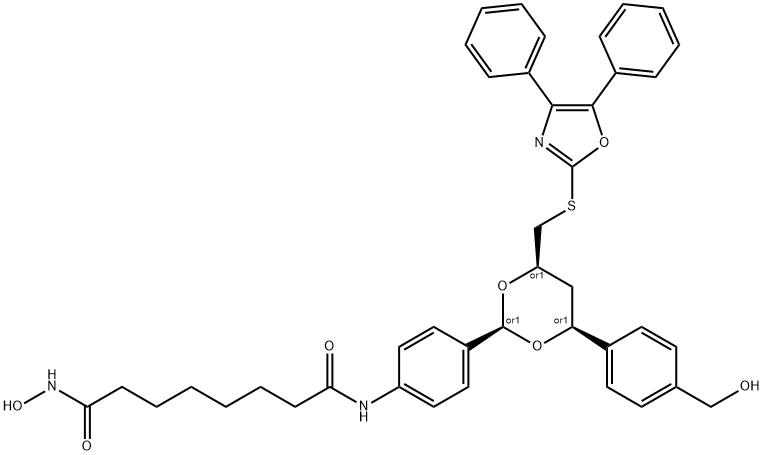 |
| | Tubacin Chemical Properties |
| Melting point | 155 - 157°C | | storage temp. | -20°C | | solubility | DMSO: ≥10mg/mL | | form | powder | | color | white to tan | | InChIKey | BHUZLJOUHMBZQY-UHHSDZQINA-N | | SMILES | C(NC1=CC=C([C@H]2O[C@@H](CSC3=NC(C4=CC=CC=C4)=C(C4=CC=CC=C4)O3)C[C@@H](C3=CC=C(CO)C=C3)O2)C=C1)(=O)CCCCCCC(NO)=O |&1:6,8,29,r| |
| | Tubacin Usage And Synthesis |
| Found | Tubacin was first discovered by Stuart Schreiber's laboratory through a multidimensional chemical genetic screen of 7392 small molecules. Tubacin induces acetylation of α-tubulin (EC50 = 2.9 μM) without causing a marked increase in acetylation of histones (EC50 = 217 μM). Also, tubacin does not induce HSP90 acetylation. Tubacin does not affect global histone deacetylation, gene expression profiling, or cell cycle progression.
| | Description | Tubacin: an HDAC6 Selective Inhibitor.
Tubacin (tubulin acetylation inducer) is a highly potent and selective, reversible, cell-permeable inhibitor of HDAC6 (IC50=0.004μM). It dose-dependently boosted the release of influenza A virus (IAV) progeny through the increase of acetylated microtubules to effectively move viral components to the plasma membrane. It could reduce the Replication of the Japanese Encephalitis Virus via the Decrease of Viral RNA Synthesis. Tubacin was demonstrated as a host-targeting agent with preventive and therapeutic activities against the Japanese encephalitis virus. Concentration in cell culture experiments typically ranges from 2-50μM[1]. | | Uses | Tubacin is tubulin acetylation inducer that selectively inhibits histone deacetylase (HDAC6). | | Definition | ChEBI: N-[4-[(2R,4R,6S)-4-[[(4,5-diphenyl-2-oxazolyl)thio]methyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]phenyl]-N'-hydroxyoctanediamide is a member of 1,3-oxazoles. | | Biochem/physiol Actions | Tubacin triggers the release of reactive oxygen species and mediates caspase-3-independent apoptosis of Epstein-Barr virus (EBV)-positive Burkitt lymphoma cells. It suppresses the proliferation of acute lymphoblastic leukemia (ALL) cells and enhances the effect of chemotherapy to treat ALL cells. Tubacin prevents the epileptic activity and neuronal migration defects caused due to lowered expression of Srpx2 in rats. | | target | HDAC6 | | References | [1] Chien-Yi Lu. “Tubacin, an HDAC6 Selective Inhibitor, Reduces the Replication of the Japanese Encephalitis Virus via the Decrease of Viral RNA Synthesis.” International Journal of Molecular Sciences 18 5 (2017).
|
| | Tubacin Preparation Products And Raw materials |
|