ethyl-2,2-dimethyl-7-bromoheptanoate manufacturers
|
| | ethyl-2,2-dimethyl-7-bromoheptanoate Basic information |
| Product Name: | ethyl-2,2-dimethyl-7-bromoheptanoate | | Synonyms: | ethyl-2,2-dimethyl-7-bromoheptanoate;7-Bromo-2,2-dimethylheptanoic acid ethyl ester;ETC-1002 intermediate 2;7-bromo-2,2-dimethylheptanoate;2,14-dimethyl-8-oxopentadecane-2,7,9,14-tetracarboxylic acid;Heptanoic acid, 7-bromo-2,2-dimethyl-, ethyl ester;Bempedoic Acid Impurity 9;Bempedoic acid intermediate | | CAS: | 123469-92-1 | | MF: | C11H21BrO2 | | MW: | 265.19 | | EINECS: | | | Product Categories: | | | Mol File: | 123469-92-1.mol | 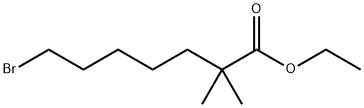 |
| | ethyl-2,2-dimethyl-7-bromoheptanoate Chemical Properties |
| Boiling point | 106-108 °C(Press: 0.01 Torr) | | density | 1.169±0.06 g/cm3(Predicted) | | vapor pressure | 0.0±0.6 mmHg at 25°C | | refractive index | 1.463 | | Fp | 146.4±13.0 °C | | InChI | InChI=1S/C11H21BrO2/c1-4-14-10(13)11(2,3)8-6-5-7-9-12/h4-9H2,1-3H3 | | InChIKey | SYRIIFZUZOIRNU-UHFFFAOYSA-N | | SMILES | C(OCC)(=O)C(C)(C)CCCCCBr | | LogP | 3.92 |
| | ethyl-2,2-dimethyl-7-bromoheptanoate Usage And Synthesis |
| Description | Ethyl 7-bromo-2,2-dimethylheptanoate is an important raw material in the synthesis route of bepetidic acid. Bempedoic acid, whose chemical name is 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid, also known as ETC-1002, is a new type of lipid developed by the American EsperionTherapeutic company Quality regulation small molecule drugs. As one of the company's main drug candidates, its targets are liver adenosine triphosphate-citrate lyase (ACL) and adenosine monophosphate-activated protein kinase (AMPK). | | Uses | Ethyl-2,2-dimethyl-7-bromoheptanoate can be used as an intermediate in organic synthesis, mainly used in laboratory research and development and chemical production processes. | | Synthesis | 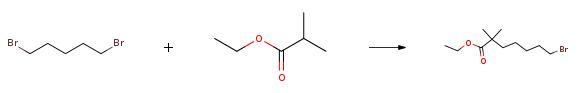
The specific embodiment of the present invention is as follows: prepare 19.4g (167mmol) of ethyl isobutyrate in 216mL solution of tetrahydrofuran with a mass concentration of 10%, connect to metering pump 1; prepare 84mL (168mmol) of 2.0M n-butyllithium solution, connect to meter Pump 2; configure 1,5-dibromopentane 80.0g (348mmol) tetrahydrofuran 100mL solution, mass concentration is 63%, connected to metering pump 3.Configure 300mL of 1N hydrochloric acid solution and connect to metering pump 4.Set continuous flow precooler 1, the circulating temperature is 0, and reach stability; set continuous flow precooler 2, the circulating temperature is 0, and reach stability; set continuous flow precooler 3, and the circulating temperature is 0, and reach stability; set continuous flow precooler 4, cycle temperature is 0, and reach stability;Set the flow rate of metering pump 1 to 65.0ml/min, set the flow rate of metering pump 2 to 25.0ml/min, set the flow rate of metering pump 3 to 30.0ml/min, and set the flow rate of metering pump 4 to 90.0ml/min.Turn on the metering pump 1 and metering pump 2 at the same time. After 60s of operation, turn on the metering pump 3, and after 60s of operation, turn on the metering pump 4 to collect the outflowing reaction liquid in the storage tank.After the operation is completed, separate the liquids, collect the upper organic phase, concentrate under reduced pressure at P=-0.08MPa, T=45??C to remove low-boiling solvents, and then rectify under reduced pressure at P=5mmHg and T=70??C to obtain 38.4 g.The obtained product is a colorless liquid with a GC purity of 98.6% and a yield of 87%. |
| | ethyl-2,2-dimethyl-7-bromoheptanoate Preparation Products And Raw materials |
|