- p-Tolyl Acetate
-

- $0.00 / 1KG
-
2025-04-27
- CAS:140-39-6
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 500kgs/month
- P-TOLYL ACETATE
-

- $45.00 / 1kg
-
2025-04-27
- CAS:140-39-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
- P-TOLYL ACETATE
-

- $0.00 / 1kg
-
2024-07-22
- CAS:140-39-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10ton
|
| | P-TOLYL ACETATE Basic information | | Aroma |
| Product Name: | P-TOLYL ACETATE | | Synonyms: | P-ACETOXYTOLUENE;P-CRESYL ACETATE;P-TOLYL ACETATE;4-Acetoxytoluene;Narceol;Paracresyl acetate;paracresylacetate;p-Cresol acetate | | CAS: | 140-39-6 | | MF: | C9H10O2 | | MW: | 150.17 | | EINECS: | 205-413-1 | | Product Categories: | Organics | | Mol File: | 140-39-6.mol | 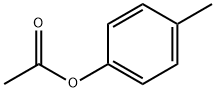 |
| | P-TOLYL ACETATE Chemical Properties |
| Melting point | 48.5 °C | | Boiling point | 210-211 °C(lit.) | | density | 1.047 g/mL at 25 °C(lit.) | | vapor pressure | 21.864Pa at 25℃ | | FEMA | 3073 | P-TOLYL ACETATE | | refractive index | n20/D 1.501(lit.) | | Fp | 194 °F | | storage temp. | Sealed in dry,Room Temperature | | solubility | Insoluble in water | | form | powder to lump to clear liquid | | Specific Gravity | 1.052 (20/4℃) | | color | White or Colorless to Almost white or Almost colorless | | Odor | at 1.00 % in dipropylene glycol. narcissus phenolic animal | | Odor Type | floral | | Water Solubility | 1.195g/L at 25℃ | | JECFA Number | 699 | | LogP | 2.11 at 25℃ | | CAS DataBase Reference | 140-39-6(CAS DataBase Reference) | | NIST Chemistry Reference | Acetic acid, 4-methylphenyl ester(140-39-6) | | EPA Substance Registry System | Acetic acid, 4-methylphenyl ester (140-39-6) |
| Hazard Codes | Xn | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36/37/39 | | RIDADR | NA 1993 / PGIII | | WGK Germany | 2 | | RTECS | AJ7570000 | | HS Code | 2915.39.3500 | | toxicity | The acute oral LD50 in rats was reported as 1.9 (1.12-3.23) g/kg (Denine, 1973). The acute dermal LD50 in rabbits was reported as 2.1 (1.24-3.57) g/kg (Denine, 1973). |
| | P-TOLYL ACETATE Usage And Synthesis |
| Aroma | P-TOLYL ACETATE has pungent Lily-like odor, penetrating, floral,
Narcissus-like in high dilution, Horse-like,
urine-like undertones in higher concentration.
Pleasant notes are obtained normally at concentrations below 1% in the perfume creation,
but the material is still capable of totally
ruining a composition when the acetate is
applied in combination with certain fragrance
notes or single chemicals.
Finds some use in flavor compositions for
its intensely floral power, which can support
fruity notes, nut-like aromas, etc.
The concentration in the finished product is
usually about 0.5 to 10 ppm, but may reach
220 ppm in chewing gums. | | Chemical Properties | Colorless liquid, volatile with steam. Poorly
soluble in water, miscible with alcohol and
oils. soluble in Propylene glycol. Sp.Gr. 1.05.
B.P. 212°C. | | Chemical Properties | p-Tolyl acetate has a strong, floral odor (narcissus) with a characteristic honey-like flavor | | Occurrence | Reported as a constituent of the essential oils of wallflower, cananga and ylang-ylang. | | Uses | Perfumery, flavoring. | | Uses | p-Tolyl acetate has been used in the total synthesis of (?)-incrustoporin. | | Preparation | By acetylation of p-cresol. | | Definition | ChEBI: Tolylacetate is a member of phenols and a benzoate ester. | | Aroma threshold values | Detection: 25 ppb | | Synthesis Reference(s) | The Journal of Organic Chemistry, 43, p. 2417, 1978 DOI: 10.1021/jo00406a025 | | General Description | The Fries rearrangement of p-tolyl acetate has been investigated using the H-form of various zeolites as catalysts. Mechanism of photo-Fries rearrangement of p-tolyl acetate has been studied. | | Flammability and Explosibility | Not classified | | Safety Profile | Moderately toxic by
ingestion and skin contact. Combustible
liquid. When heated to decomposition it
emits toxic smoke and irritating fumes. |
| | P-TOLYL ACETATE Preparation Products And Raw materials |
|