アバレリックス
|
- ¥128700
- 化學(xué)名: アバレリックス
- 英語名: Abarelix
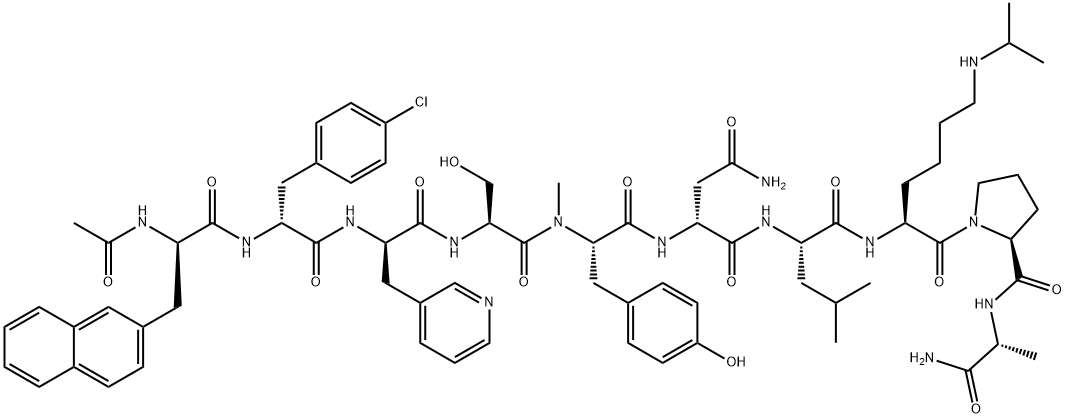
- 別名:アバレリックス;アバレリクス;(2R)-N-[(1S)-1-{[(2S)-1-[(2S)-2-{[(1R)-1-カルバモイルエチル]カルバモイル}ピロリジン-1-イル]-1-オキソ-6-[(プロパン-2-イル)アミノ]ヘキサン-2-イル]カルバモイル}-3-メチルブチル]-2-[(2S)-2-[(2S)-2-[(2R)-2-[(2R)-3-(4-クロロフェニル)-2-[(2R)-2-アセトアミド-3-(ナフタレン-2-イル)プロパンアミド]プロパンアミド]-3-(ピリジン-3-イル)プロパンアミド]-3-ヒドロキシ-N-メチルプロパンアミド]-3-(4-ヒドロキシフェニル)プロパンアミド]ブタンジアミド;N-アセチル-3-(2-ナフチル)-D-Ala-4-クロロ-D-Phe-3-(3-ピリジニル)-D-Ala-L-Ser-N-メチル-L-Tyr-D-Asn-L-Leu-N6-イソプロピル-L-Lys-L-Pro-D-Ala-NH2;PPI-149
- CAS番號: 183552-38-7
- 分子式: C72H95ClN14O14
- 分子量: 1416.06
- EINECS:1592732-453-0
- MDL Number:MFCD06407663
1物価
選択條件:
ブランド
- 富士フイルム和光純薬株式會社(wako)
パッケージ
- 5mg
- 生産者富士フイルム和光純薬株式會社(wako)
- 製品番號W01TRCA107225
- 製品説明アバレリックス
- 英語製品説明Abarelix Trifluoroacetate Salt
- 包裝単位5mg
- 価格¥128700
- 更新しました2024-03-01
- 購入
| 生産者 | 製品番號 | 製品説明 | 包裝単位 | 価格 | 更新時間 | 購入 |
|---|---|---|---|---|---|---|
| 富士フイルム和光純薬株式會社(wako) | W01TRCA107225 | アバレリックス Abarelix Trifluoroacetate Salt |
5mg | ¥128700 | 2024-03-01 | 購入 |
プロパティ
沸點 :1688.4±65.0 °C(Predicted)
比重(密度) :1.286±0.06 g/cm3(Predicted)
貯蔵溫度 :Store at -20°C
溶解性 :Methanol (Slightly), Water (Slightly)
酸解離定數(shù)(Pka) :9.82±0.15(Predicted)
外見 :Solid
色 :White to Off-White
InChIKey :AIWRTTMUVOZGPW-HSPKUQOVSA-N
CAS データベース :183552-38-7
比重(密度) :1.286±0.06 g/cm3(Predicted)
貯蔵溫度 :Store at -20°C
溶解性 :Methanol (Slightly), Water (Slightly)
酸解離定數(shù)(Pka) :9.82±0.15(Predicted)
外見 :Solid
色 :White to Off-White
InChIKey :AIWRTTMUVOZGPW-HSPKUQOVSA-N
CAS データベース :183552-38-7
安全情報
| 絵表示(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| 注意喚起語: | ||||||||
| 危険有害性情報: |
|
|||||||
| 注意書き: |
|
説明
Abarelix is an antagonist of the gonadotropin releasing-hormone (Gn RH) receptor, and it was launched as an intramuscular injection for the palliative treatment of advanced symptomatic prostate cancer. Hormonal therapy of prostate cancer is based on the modulation of testosterone to achieve medical castration levels. The inhibition of GnRH activity causes the suppression of luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion, thereby reducing the secretion of testosterone by the testes. Abarelix is the first GnRH antagonist to reach its market. Hormonal therapy with GnRH agonists such as leuprolide, buserelin, and goserelin has been in use for over two decades. Drugs of this type achieve the suppression of LH and FSH by a feedback inhibition mechanism, which involves an initial rise in the LH and FSH levels and, consequently, in the levels of testosterone. A testosterone surge may induce a clinical tumor flare that worsens cancer-related symptoms. This phenomenon is observed in 4–33% of patients receiving a GnRH agonist. A GnRH antagonist such as abarelix acts by direct inhibition of LH and FSH secretion, which avoids the initial surge in serum testosterone concentrations. Abarelix is a decapeptide, and it is prepared by a typical coupling cycle for peptide synthesis using Boc-amino acids and a methylbenzhydrylamine (MBHA) resin. Abarelix has high binding affinity for GnRH receptor (Kd=0.1 nM). Following intramuscular administration of a 100 mg dose, abarelix is absorbed slowly with a Cmax of 43.4 ng/mL observed approximately 3 days after the injection and has a half-life of about 13 days. The apparent volume of distribution is over 4000 L, suggesting extensive distribution. Abarelix has high protein binding (96–99%), and it is primarily metabolized via hydrolysis of peptide bonds. Following a dose of 15 μg/kg in humans, approximately 13% of abarelix is recovered unchanged in the urine, with no detectable metabolites. The renal clearance of abarelix is 14.4 L/day following a 100 mg dose. Two randomized, open label, comparative clinical trials involving 348 patients demonstrated the efficacy of abarelix versus a GnRH agonist (leuprolide) as well as a combination of GnRH agonist and anti-androgen (leuprolide+bicalutamide). In these trials, both abarelix and the comparators reduced testosterone to medical castration levels (<50 ng/dL) by day 29 of therapy in 94–98% of the patients. However, a significant difference was observed between the two groups for the occurrence of testosterone surge (0% in the abarelix group versus 82% in the GnRH agonist group) and for the rapidity of attaining castration levels (72% versus 0% on day 8 in the abarelix and GnRH agonist groups, respectively). Both groups maintained medical castration levels of testosterone with similar efficacy between days 29 and 85 of treatment. Abarelix was generally well tolerated in these trials. Approximately 3% of the patients experienced an immediate-onset allergic reaction. Other adverse events were similar to comparator controls and included hot flushes, sleep disturbance, pain, and breast enlargement. The recommended dosage of abarelix is 100 mg intramuscular injection on days 1, 15, and 29 of therapy, and every 4 weeks thereafter.関連製品価格
コートロシン LH-RH オクトレオチド·酢酸塩 副腎皮質(zhì)刺激ホルモン放出因子 Β-アミロイドペプチド (1-42), ヒト H-SER-VAL-SER-GLU-ILE-GLN-LEU-MET-HIS-ASN-LEU-GLY-LYS-HIS-LEU-ASN-SER-MET-GLU-ARG-VAL-GLU-TRP-LEU-ARG-LYS-LYS-LEU-GLN-ASP-VAL-HIS-ASN-PHE-OH : PTH (1-34) (ヒト) トリプトレリン 8-オルニチンバソプレッシン 酢酸ソマトレリン プラルモレリン ピトレッシン 酢酸ゴセレリン 3-メルカプト(1)プロピオニル-L-Tyr-L-Phe-L-Gln-L-Asn-L-Cys(1)-L-Pro-D-Arg-Gly-NH2·酢酸塩 アセチルペプスタチン 酢酸バプレオチド 酢酸トリプトレリン 酢酸ナファレリン エプチフィバチド 二酢酸アルギニンバソプレッシン ランレオチド酢酸塩関連商品価格
- コートロシン
¥8200-281000 - LH-RH
¥2700-95000 - オクトレオチド·酢酸塩
¥21500-407100
供給者とメーカー
Apextide Co Ltd
Hebei Yanxi Chemical Co., Ltd.
Zibo Hangyu Biotechnology Development Co., Ltd
Anhui Ruihan Technology Co., Ltd
Nantong Guangyuan Chemicl Co,Ltd
Hangzhou Hyper Chemicals Limited
Henan Tianfu Chemical Co.,Ltd.
Hangzhou FandaChem Co.,Ltd.
career henan chemical co
SHANDONG ZHI SHANG CHEMICAL CO.LTD




