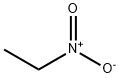
????? C??? ??, ??, ??
??? ??
Nitroethane is a colorless, oily liquid with a
mild, fruity odor. The Odor Threshold is 163 ppm.
??? ??
Colorless, very flammable liquid with a fruity odor. Odor threshold concentration is 2.1 ppm
(quoted, Amoore and Hautala, 1983). Concentrated mixtures usually contain 98 wt % nitroethane
and 2 wt % moisture.
??
Solvent, artificial fingernail glue remover; in organic syntheses. Experimentally as liquid propellant.
?? ??
Industrial production of nitroethane is by vapor-phase nitration of propane with
nitric acid, followed by fractional distillation (Baker and Bollmeier 1978). U.S.
production was greater than 454 kg in 1975 (HSDB 1988).
??
ChEBI: A nitroalkane that is ethane substituted by a nitro group.
?? ??
A colorless oily liquid with a pleasant odor. Flash point of 82°F. Decomposes above 350°F. Density 1.052 g / cm3. Vapors much heavier than air. and insoluble in water. Vapors may irritate skin, eyes and mucous membranes. Produces toxic oxides of nitrogen during combustion. Used as a propellant and as a solvent.
??? ?? ??
Highly flammable. Water soluble.
?? ???
The nitroparaffins, nitromethane, nitropropane, etc. form salts with inorganic bases such as calcium hydroxide. The dry salts are explosive [Chem. Eng. News 30:2344. 1952]. Nitroethane and other nitro compounds are mild oxidizers and should not be heated with easily oxidizable hydrocarbons under confinement [Chem. Eng. News 30:2344. 1940].
???
Moderate fire risk.Upper respiratory tract
irritant, central nervous system impairment, and
liver damage.
????
Nitroethane is irritating to the eyes and mucous membranes (HSDB 1988),
however, there have been no reports of serious toxic effects of the chemical in
humans.
????
Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fire.
?? ??
Nitroethane is used as a solvent for cellulose esters, vinyl and other resins and
waxes and as a solvent in batteries (Baker and Bollmeier 1978).
Safety Profile
Poison by
intraperitoneal route. Moderately toxic by
ingestion. Mildly toxic by inhalation. Causes
injury to liver and hdneys. An eye and
mucous membrane irritant. Flammable
liquid when exposed to heat, sparks, flame,
or oxidizers. To fight fire, use alcohol foam,
CO2, dry chemical; water can blanket fire.
Incompatible with Ca(OH)2, hydrocarbons,
hydroxides, inorganic bases, KOH, NaOH,
metal oxides, Explodes when heated. When heated to decomposition it emits toxic
fumes of NOx. See also NITRO
COMPOUNDS.
??? ??
Nitroethane is used as solvent for
polymers, cellulose esters; vinyl, waxes, fats, dyestuffs, and
alkyd resins; as a stabilizer. It has been used as a rocket
propellant. It is used as an intermediate in pharmaceutical
manufacture and in pesticide manufacture.
????
Chemical/Physical. 2-Nitroethane will not hydrolyze because it does not contain a hydrolyzable
functional group.
?? ??
UN2842 Nitroethane, Hazard Class: 3; Labels:
3-Flammable liquid.
Purification Methods
Purify it as described for nitromethane below. A spectroscopic impurity can be removed by shaking it with activated alumina, decanting and distilling it rapidly. [Beilstein 1 IV 170.]
? ???
A nitroparaffin, nitroethane forms explosive
mixture with air. Explodes when heated or when
shocked; in confined area, with elevated temperatures. A
strong reducing agent. Violent reaction with oxidizers,
hydrocarbons, other combustibles; amines, metal oxides.
Forms shock-sensitive compounds with strong acids; strong
alkalis. Attacks some plastics and coatings.
??? ??
Incineration: large quantities
of material may require nitrogen oxide removal by catalytic
or scrubbing processes.
????? ?? ?? ? ???
???
?? ??