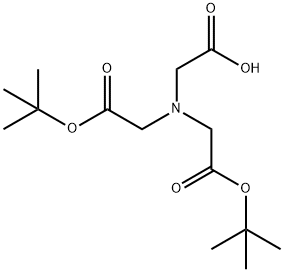
(BIS-TERT-BUTOXYCARBONYLMETHYL-AMINO)-ACETIC ACID synthesis
- Product Name:(BIS-TERT-BUTOXYCARBONYLMETHYL-AMINO)-ACETIC ACID
- CAS Number:171557-31-6
- Molecular formula:C14H25NO6
- Molecular Weight:303.35
![Glycine, N,N-bis[2-(1,1-dimethylethoxy)-2-oxoethyl]-, phenylmethyl ester](/CAS/20210305/GIF/171557-57-6.gif)
171557-57-6
0 suppliers
inquiry

171557-31-6
57 suppliers
$13.00/250mg
Yield:171557-31-6 97%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; for 2 h;
Steps:
Synthesis of 12
Compound 11 (477 mg, 1.17 mmol) was used as a starting material. By the same synthetic procedure as that for 9, 12 (335 mg, 97%) was obtained as a pale yellow oil.
References:
Takahira, Ikuko;Fuchida, Hirokazu;Tabata, Shigekazu;Shindo, Naoya;Uchinomiya, Shohei;Hamachi, Itaru;Ojida, Akio [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 13,p. 2855 - 2858] Location in patent:supporting information
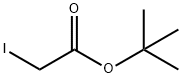
49827-15-8
65 suppliers
$75.00/1g

171557-31-6
57 suppliers
$13.00/250mg
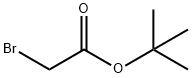
5292-43-3
453 suppliers
$10.00/5g

171557-31-6
57 suppliers
$13.00/250mg