????????
|
|
???????? ??
- ???
- -89.5 °C
- ?? ?
- 82 °C(lit.)
- ??
- 0.785 g/mL at 25 °C(lit.)
- ?? ??
- 2.1 (vs air)
- ???
- 33 mm Hg ( 20 °C)
- ???
- n
20/D 1.377(lit.)
- ???
- 53 °F
- ?? ??
- Store at +5°C to +30°C.
- ???
- ?: ???(???)
- ?? ?? (pKa)
- 17.1(at 25℃)
- ??? ??
- ??? ??
- Specific Gravity
- approximate 0.785(20/20℃)(Ph.Eur.)
- ??
- ???
- ????
- 0.546
- ??
- ???????; ???? ?? ???; ???? ??? ??? ???; ???.
- ??????(pH)
- 7.0 (20°C)
- ???? ??
- synthetic
- ?? ??
- ??? ???
- Odor Threshold
- 26ppm
- ????
- 2-13.4%(V)
- ???
- ?? ??
- ???
- -89.5℃
- ?? ??(λmax)
- λ: 260 nm Amax: 0.02
λ: 280 nm Amax: 0.01
- Merck
- 14,5208
- JECFA Number
- 277
- BRN
- 635639
- ?? ??
- TLV-TWA 980 mg/m3 (400 ppm); STEL 1225 mg/m3 (500 ppm) (ACGIH); IDLH 12,000 ppm (NIOSH).
- Dielectric constant
- 18.0(Ambient)
- ???
- ??? ??
- LogP
- 0.050
- ????
- 21.3mN/m at 293.15K
- CAS ??????
- 67-63-0(CAS DataBase Reference)
- IARC
- 3 (Vol. 15, Sup 7, 71) 1999
- Absorption
- in accordance
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi,F,Xn | ||
|---|---|---|---|
| ?? ???? ?? | 11-36-67-40-10-36/38 | ||
| ????? | 7-16-24/25-26-36/37 | ||
| ????(UN No.) | UN 1219 3/PG 2 | ||
| OEB | A | ||
| OEL | TWA: 400 ppm (980 mg/m3), STEL: 500 ppm (1225 mg/m3) | ||
| WGK ?? | 1 | ||
| RTECS ?? | NT8050000 | ||
| F ?????? | 3-10 | ||
| ?? ?? ?? | 750 °F | ||
| TSCA | Yes | ||
| HS ?? | 2905 12 00 | ||
| ?? ?? | 3 | ||
| ???? | II | ||
| ?? ?? ??? | 67-63-0(Hazardous Substances Data) | ||
| ?? | LD50 orally in rats: 5.8 g/kg (Smyth, Carpenter) | ||
| IDLA | 2,000 ppm [10% LEL] | ||
| ???? ?? | KE-29363 |
???????? C??? ??, ??, ??
??
Iso-Propyl Alcohol (IPA) 2-Propanol, sec-Propyl alcohol, ?? ??? ???, ?????? ?? ??; ?? ??? ?? ??? ??? ??????. ??? 1- ????? ?? ??????. ???? IPA? ?? ?? ?? ?? ? ??? ???????.??
?????? ???(isopropyl alcohol) ?? ???-2-?(propan-2-ol), 2-????(2-propanol)? ??? C3H8O? ?? ????, ????, ???? ??? ????. ??? ??? ???? ?? ??? ??? ?? ?? ???? ??? ?? ???, LCD ? IT ?? ????? ?? ????, ???, ?? ?? ???? ???? ?? ???? ?? ?? ????.??
?? ??? ??? (Iso-Propyl Alcohol, IPA)? ???, ?? ? ?? ?? ??, ???, ??, ?? ??, ?? ??, ???, ??, ?? ???? ??? ??? ?? ?????. ?? ?? ??? ??? ? ??? ?? ??, ? ?? ??, ?? ? ?? ???, ???, ???, ??? ? ?? ???, ?? ?? ?? ???? ??? ? ?? ?? ? ?? ?? ?? ? ???? ?????.?? ??
?? ??? ??? (Iso-Propyl Alcohol, IPA)? ???, ?? ? ?? ?? ??, ???, ??, ?? ??, ?? ??, ???, ??, ?? ???? ??? ??? ?? ?????.????
? (1) ??? : ? ?? 10mL? ? 40mL? ??? ?? 1?? ? ? ?? ??? ??? ??? ??.
??(2) ??(?????) : ? 100mL? ???????? 2??? ??? ??? 30?? ?? ??? ??? ??? 0.01N ????????? ?? ?? ? ?? 50mL(? 39g? ???? ?)? ?? ???. ?? ?? ??? ??? ??? 0.01N ?????????? ??? ?, ? ???? 0.7mL ????? ??.
??(3) ? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(4) ???? : ? ??? ?? ? ?????? ?? ??? ??? ?, 81.3~83.3℃?? 95%(v/v) ??? ????? ??.
??(5) ????? : ? ?? 125mL(? 100g? ???? ?)? ????? ?????? 105℃?? 30?? ??? ?, ? ?? 10ppm ????? ??.
??(6) ?????? ???? ?? : ? ?? 50mL? ??? ?? 50mL ???? ?? 0.1N ???????? 0.25mL? ?? ?? 10?? ??? ?, ?? ??? ??? ????? ?? ??.
??(7) ?? : ? ??? ??? 0.784~0.788??? ??.
??(8) ??? : ? ??? ??? 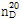 ? 1.374~1.380??? ??.
? 1.374~1.380??? ??.
????
? ? ?? 2mL? ? 3mL? ???????? 1mL? ??? ???? ? ?, ?~??? ??? ???.
???
? ? ?? ? 500mg? ??? ?? ?? ?? ??? 50mL? ?? ? ? 10mL? ??? ?? ??? 100mL? ? ?? ?????? ??. ?? ?????????? ? 500mg? ??? ?? ????? ???? ??? ?? ?????? ??. ???? 4mL ? ???? 4mL? 100mL ??????? ?? ?? ?? ? ????? ??????(tert-???? ? 500mg? ??? ?? ?? ?? ??? 50mL? ?? ? ? 10mL? ??? ?? ??? 100mL? ? ?) 4mL? ??? ??? ?? ?? ??? 100mL? ? ?? ???? ? ?????? ??. ???? 1μL ? ???? 1μL? ?? ?????????? ???? ?? ???? ?? ???????? ??(%)? ???.
WS : ??????????? ??(mg)
WU : ??? ??(mg)
RU : ?????? tert-????? ????? ?? ??????? ???? ?
RS : ?????? tert-????? ????? ?? ??????? ???? ?
????????
???????????? ? : ?? 2mm, ?? 2m? ??? ?? ??????
??????????? : ????? W—HP? 3% OV—225? ?? ?
???????? ?? ? : ?????????(FID)
??????????? : 250℃
????? ???? : 200℃
??????????? : 250℃
??????????? ? ?? : ??, 30mL/min
??
Isopropanol is a clear, colorless alcohol that is used in the production of acetone and as a solvent in the manufacture of various industrial and commercial products. It is used by the public for a number of different purposes and is commonly known as rubbing alcohol. It is flammable and miscible with both water and many different organic solvents. Isopropanol can be prepared via three different methods: indirect hydration of propylene (the ‘strong acid’ method), direct hydration of propylene, and catalytic hydrogenation of acetone.??? ??
Isopropyl alcohol is a clear, colorless, mobile, volatile, flammable liquid with a characteristic, spirituous odor resembling that of a mixture of ethanol and acetone; it has a slightly bitter taste.It is miscible with water, ethyl ether, and ethyl alcohol. Isopropyl alcohol is incompatible with strong oxidizers, acetaldehyde, chlorine, ethylene oxide, acids, and isocyanates.??
Reported found in apple and cognac aromas (esterified). Also found in apple, banana, grapefruit and lime juice, melon, papaya, pear, onion, peas, rutabaga, tomato, wheat bread, cheeses, milk, boiled egg, cooked beef, pork and mutton, hop oil beer, rum, cocoa, coffee, scotch whiskey, grape wines, peanut, pecan, soybean, honey, beans, plum brandy, walnut, crab, clam, prickly pear and clary sage.?? ??
Isopropyl alcohol may be prepared from propylene; by the catalytic reduction of acetone, or by fermentation of certain carbohydrates.??
ChEBI: Isopropyl Alcohol is a secondary alcohol that is propane in which one of the hydrogens attached to the central carbon is substituted by a hydroxy group. It is an isomer of propyl alcohol with antibacterial properties.?? ??
Isopropyl Alcohol is used in a variety of applications including as a solvent for industrial processes and coating; as a component in cleaning, car care and deicing products; as a wetting agent for printing inks and as a feedstock in the manufacture of ester and Mogas/Luboil additives.isopropyl alcohol is a carrier, anti-bacterial, and solvent for skin care lotions. Isopropyl alcohol is made from propylene, a petroleum derivative.
When compared to ethanol, 50% less is required for nucleic acid precipitation, thus minimizing the total volume to be centrifuged for DNA or RNA recovery.
Isopropyl alcohol 70% is used as an ingredient in alcohol swabs and alcohol wipes for wound cleaning, it is found in hand sanitizers, and in ear drops to prevent swimmer's ear.
?? ??
Volatile, colorless liquid with a sharp musty odor like rubbing alcohol. Flash point of 53°F. Vapors are heavier than air and mildly irritating to the eyes, nose, and throat. Density approximately 6.5 lb / gal. Used in making cosmetics, skin and hair preparations, pharmaceuticals, perfumes, lacquer formulations, dye solutions, antifreezes, soaps, window cleaners. Sold in 70% aqueous solution as rubbing alcohol.??? ?? ??
Highly flammable. Water soluble.?? ???
Isopropyl Alcohol can react with AIR and OXYGEN over time to form unstable peroxides that can explode. Contact with 2-butanone increases the rate of peroxide formation. An explosive reaction occurs when Isopropanol is heated with (aluminum isopropoxide + crotonaldehyde). Forms explosive mixtures with trinitromethane and hydrogen peroxide. Reacts with barium perchlorate to form a highly explosive compound. Ignites on contact with dioxygenyl tetrafluoroborate, chromium trioxide and potassium-tert-butoxide. Vigorous reactions occur with (hydrogen + palladium), nitroform, oleum, COCl2, aluminum triisopropoxide and oxidizing agents. Reacts explosively with phosgene in the presence of iron salts. Incompatible with acids, acid anhydrides, halogens and aluminum . Isopropanol can react with PCl3, forming toxic HCl gas. (Logsdon, John E., Richard A. Loke., sopropyl Alcohol. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 1996.).????
Exposures to isopropyl alcohol cause irritation to the eyes and mucous membranes. Exposures to isopropyl alcohol for 3–5 min (400 ppm) caused mild irritation of the eyes, nose, and throat, and at 800 ppm these symptoms became severe. Ingestion or an oral dose of 25 mL in 100 mL of water produced hypotension, facial flushing, bradycardia, and dizziness. Ingestion in large quantities caused extensive hemorrhagic tracheobronchitis, bronchopneumonia, and hemorrhagic pulmonary edema. Prolonged skin contact with isopropyl alcohol caused eczema and sensitivity. Delayed dermal absorption is attributed to a number of pediatric poisonings that have occurred following repeated or prolonged sponge bathing with isopropyl alcohol to reduce fever. In several cases, symptoms included respiratory distress, stupor, and coma. Laboratory animals exposed to isopropyl alcohol develop poisoning with symptoms of hind leg paralysis, unsteadiness, lack of muscular coordination, respiratory depression, and stupor. Isopropyl alcohol is a potent CNS depressant, and in large doses causes cardiovascular depression.????
Isopropyl Alcohol(IPA) is highly flammable in its liquid and vapor forms and flammable atmospheres can be created at temperatures as low as 540°F /120℃ . This means that any environment where IPA is being used needs to be well ventilated. It should be kept away from heat and open flame. As the vapour is heavier than air, it may spread along the ground, so care needs to be taken that the vapour is not ignited by a distant source.Pharmaceutical Applications
Isopropyl alcohol (propan-2-ol) is used in cosmetics and pharmaceutical formulations, primarily as a solvent in topical formulations.( It is not recommended for oral use owing to its toxicity.Although it is used in lotions, the marked degreasing properties of isopropyl alcohol may limit its usefulness in preparations used repeatedly. Isopropyl alcohol is also used as a solvent both for tablet film-coating and for tablet granulation, where the isopropyl alcohol is subsequently removed by evaporation. It has also been shown to significantly increase the skin permeability of nimesulide from carbomer 934.
Isopropyl alcohol has some antimicrobial activity and a 70% v/v aqueous solution is used as a topical disinfectant. Therapeutically, isopropyl alcohol has been investigated for the treatment of postoperative nausea or vomiting.
Carcinogenicity
CD-1 mice were exposed by inhalation to 0, 500, 2500, or 5000 ppm of isopropanol vapor for 6 h/day, 5 days/week for 18 months. An additional group of mice (all exposure levels) were assigned to a recovery group that were exposed to isopropanol for 12 months and then retained until study termination at 18 months. There was no increased frequency of neoplastic lesions in any of the isopropanol-exposed animals. Nonneoplastic lesions were limited to the testes (males) and the kidney. In the testes, enlargement of the seminal vesicles occurred in the absence of associated inflammatory or degenerative changes. The kidney effects included tubular proteinosis and/or tubular dilatation. The incidence of testicular and kidney effects was not increased in the isopropanol-exposed recovery animals.????
The vast majority of isopropanol in the environment originates from manufacturing processes. Small amounts are produced by certain microbes, fungi, and yeast. The high volatility of isopropanol ensures that when it is released into the environment in any state, it eventually ends up in the atmosphere. There, it can be degraded by hydroxyl radicals or it can return to soil or water through precipitation. Its half-life in the environment is approximately 3.2 days and is highly biodegradable; bioaccumulation in plants and animals does not occur.??
Isopropyl alcohol should be stored in a cool, dry, well-ventilated area in tightly sealed containers with a proper label. Outside or detached storage is preferable. Inside storage should be a flammable liquids storage room or cabinet. Workers should not store isopropyl alcohol above 37°C (100°F). Containers of isopropyl alcohol should be protected from physical damage and contact with air, and should be stored separately from strong oxidizers, acetaldehyde, chlorine, ethylene oxide, acids, and isocyanates. Isopropyl alcohol should be transported to the nearest laboratory as quickly as possible in cool containers.? ???
Incompatible with oxidizing agents such as hydrogen peroxide and nitric acid, which cause decomposition. Isopropyl alcohol may be salted out from aqueous mixtures by the addition of sodium chloride, sodium sulfate, and other salts, or by the addition of sodium hydroxide.?? ??
Workers should wash hands and face thoroughly after handling isopropyl alcohol. Workers should wear gloves, safety glasses and a face shield, boots, apron, and a full impermeable suit is recommended if exposure is possible to a large portion of the body.Regulatory Status
Included in the FDA Inactive Ingredients Database (oral capsules, tablets, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.???????? ?? ?? ? ???
???
?? ??
???????? ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40066 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8772 | 58 |
| Deoksan General Science Co., Ltd | +82-02-957-3792 +82-02-957-3792 |
dscsi@hanmail.net | South Korea | 4 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; |
peter68@ptchemgroup.com | China | 35425 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 57505 | 58 |
| Runte International Trade Limited | 19565631292 19565631292 |
lucky@sdruntechem.com | China | 185 | 58 |
| Chongqing Soarwin Technology Co., Ltd | +86-19132938950 +86-19132938950 |
crystal@sorawin.cn | China | 360 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 |
abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5868 | 58 |








