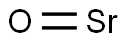???? ???
|
|
???? ??? ??
- ???
- 2430°C
- ?? ?
- 3000°C
- ??
- 4.7 g/mL at 25 °C (lit.)
- ???
- 3000°C
- ?? ??
- Sealed in dry,Room Temperature
- ???
- ????? ??? ?????. ???? ?? ?????. ???? ???? ???? ????.
- ??? ??
- ??
- Specific Gravity
- 4.7
- ??
- ???
- ???
- ??? ?????? ?????. ???? ???? ???. ?? ????? ??? Sr(OH){2}? ?????. ???? ?? ?????. ???? ???? ???? ????.
- Crystal Structure
- NaCl type
- ??
- Moisture Sensitive
- Merck
- 14,8846
- crystal system
- Cube
- Space group
- Fm3m
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.5113 0.5113 0.5113 90 90 90 0.1337
- InChI
- InChI=1S/O.Sr
- InChIKey
- UFQXGXDIJMBKTC-UHFFFAOYSA-N
- SMILES
- O=[Sr]
- CAS ??????
- 1314-11-0(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | C | ||
|---|---|---|---|
| ?? ???? ?? | 14-34 | ||
| ????? | 26-30-36/37 | ||
| ????(UN No.) | UN 3262 8/PG 2 | ||
| WGK ?? | 3 | ||
| TSCA | Yes | ||
| ?? ?? | 8 | ||
| ???? | II | ||
| ???? ?? | KE-32238 |
???? ??? C??? ??, ??, ??
??
Strontium Oxide, SrO, is a metal oxide usually produced by the reaction of strontium with oxygen. It can also be prepared by the decomposition of heated strontium carbonate, hydroxide, or nitrate and is used in manufacturing other strontium salts, in pigments, soaps and greases, and as a drying agent. It is commonly used in the glass, ceramics, and electronic industries and has other specific uses, such as fuel cells and sputtering target material. If burn strontium under general conditions with air, strontium oxide and strontium nitride will form. Strontium oxide was once required by law as the material used on old TV face-plates to block harmful X-ray emissions. BaO and SrO were reported for the transesterification of palm oil for the production of biodiesel. The reaction was conducted under ultrasound conditions to ensure the effective production of biodiesel.??? ??
white to grayish white; reacts with water, forming Sr(OH)2, with evolution of heat; enthalpy of fusion 75.00kJ/mol [MER06] [CRC10]??? ??
Strontium oxide or strontia, SrO, is formed when Sr metal reacts with oxygen:2Sr+ O2→2SrO
Burning strontium in air results in a mixture of strontium oxide and strontium nitride. It also forms from the decomposition of strontium carbonate SrCO3. It is a strongly basic oxide. It reacts with moisture forming the hydroxide and with carbon dioxide in the air to form the carbonate.
??
SrO is also used in medicine, pyrotechnics, pigments, greases, soaps, and as a chemical intermediate. It is also produced as high-purity strontium oxide rotatable sputtering targets with the highest possible density and smallest possible average grain sizes for use in semiconductor, photovoltaic, and coating applications by chemical vapor deposition and physical vapor deposition and optical applications.One of the reasons why rare metals are restrained from wide use in production is their high cost.???? ??? ?? ?? ? ???
???
?? ??
???? ??? ?? ??
???( 208)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Alfa Chemistry | |
Info@alfa-chemistry.com | United States | 24072 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3883 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29857 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9636 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17357 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10244 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
???? ??? ?? ??:
???? ???? ???? ??? ???? FERRITE ???? ???? ?? ?? ???? ?? ???? ??? ???? ??????? ?? ?? ????, ?? ??? ????, ??????, ????? Boric acid, solid soln. with barium oxide, calcium oxide and strontium oxide, lead and manganese-doped
TITANIUM DIOXIDE
Tetraterbium heptaoxide
Praseodymium oxide
Rhodium oxide
MANGANESE (III) OXIDE
Europium Oxide
COPPER CHROMITE
Cobalt oxide
Gadolinium oxide