??????? C??? ??, ??, ??
??
?? ??? ??? ?? ?? ?? ??? ??????. ?? ???? ??? ?? ???? ??, ??? ? ?? ??? ?? ?????. ??? ?? ??, ??? ??? ???, ?? ???, ?? ????, ?? ??? ? ?? ???? ????.
??
??? ??, ?? ?? ? ???, ?? ??? ??? ??; ???; ???; ???; ?? ???; ???; ?? ? ??.
??, ?? ? ??
?? ? ??? ??? ?. ???? ???? ??? ??? ?? ??? ??????.
??? ??
Pentaerythritol is a white crystalline solid.
Odorless.
??
In the manufacture of pentaerythritol
tetranitrate; alkyd resins in surface-coating compositions;
pentaerythritol triacrylate and protective
coatings; insecticides; pharmaceuticals
??
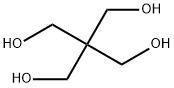
pentaerythritol: white crystallinecompound, C(CH
2OH)
4; m.p.260°C; b.p. 276°C (30 mmHg). It isused in making the explosive pentaerythritoltrinitrate and in producingresins and other organicproducts.
?? ??
Odorless white solid. Sinks and mixes slowly with water.
??? ?? ??
Water soluble.
?? ???
Pentaerythritol is an alcohol. Pentaerythritol is incompatible with the following: Organic acids, oxidizers [Note: Explosive compound is formed when a mixture of PE & thiophosphoryl chloride is heated.] .
?? ???
The four primary hydroxyl groups undergo the normal reactions of OH groups. Pentaerythritol is oxidized to tris (hydroxymethyl)acetic acid by air in the presence of platinum or palladium.
????
Non-toxic; no symptoms likely
Safety Profile
Mildly toxic by ingestion. A nuisance dust. Flammable from heat or flame or oxidizers. Wxtures with thiophosphoryl chloride react when heated to form a product that ignites and then explodes on contact with air. Used in coatings, stabdizers, explosives, P.E.T.N resins, drugs, insecticides, and lubricants When heated to decomposition it emits acrid smoke and irritating fumes
??? ??
Pentaerythritol is used in coatings and
stabilizers; in the formation of alkyd resins and varnishes.
It is used as an intermediate in the manufacture of plasticizers,
explosives (PETN),and pharmaceuticals.
?? ??
UN1987 Alcohols, n.o.s., Hazard Class: 3;
Labels: 3-Flammable liquid.
Purification Methods
Reflux pentaerythritol with an equal volume of MeOH, then cool, and the precipitate is collected and dried at 90o. It can also be crystallised from dilute aqueous HCl. After sublimation under high vacuum at 200o it has m 265.5o. Its solubility in H2O is 10%. [Beilstein 18 III 2361, 1 IV 2812.]
? ???
Dust may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong acids, organic acids,
strong bases. Aquesous solution is acidic. Explosive
compound is formed when a mixture of Pentaerythritol and
thiophosphoryl chloride (CAS 3892-91-0) is heated.
??????? ?? ?? ? ???
???
?? ??