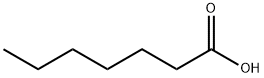
??? ?
|
|
??? ? ??
- ???
- -10.5 °C (lit.)
- ?? ?
- 223 °C (lit.)
- ??
- 0.918 g/mL at 25 °C (lit.)
- ?? ??
- 4.5 (vs air)
- ???
- <0.1 mm Hg ( 20 °C)
- ???
- n
20/D 1.4221(lit.)
- ???
- >230 °F
- ?? ??
- Store below +30°C.
- ???
- ?: 15°C?? ???0.2419g/100ml
- ?? ?? (pKa)
- 4.89(at 25℃)
- ??? ??
- ??
- ??
- ???? ?????
- ??
- ???? ??? ? 1.00%. ?? ??? ?? ?? ?
- ?? ??
- ???? ??
- ???? ??
- synthetic
- ????
- 10.1%
- ???
- 0.24g/100mL(15℃)
- Merck
- 14,4660
- JECFA Number
- 96
- BRN
- 1744723
- Dielectric constant
- 2.5(22℃)
- ???
- ????. ?? ???, ??, ???? ???? ????. ?? ??. ????? ??????.
- InChIKey
- MNWFXJYAOYHMED-UHFFFAOYSA-N
- LogP
- 2.54 at 20℃
- CAS ??????
- 111-14-8(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | C | ||
|---|---|---|---|
| ?? ???? ?? | 34 | ||
| ????? | 26-28-36/37/39-45-28A | ||
| ????(UN No.) | UN 3265 8/PG 3 | ||
| WGK ?? | 1 | ||
| RTECS ?? | MJ1575000 | ||
| ?? ?? ?? | 380 °C | ||
| TSCA | Yes | ||
| HS ?? | 2915 90 70 | ||
| ?? ?? | 8 | ||
| ???? | III | ||
| ?? ?? ??? | 111-14-8(Hazardous Substances Data) | ||
| ?? | LD50 i.v. in mice: 1200±56 mg/kg (Or, Wretlind) | ||
| ???? ?? | KE-18284 |
??? ? C??? ??, ??, ??
??
Heptanoic acid, also called enanthic acid, is an organic compound composed of a seven - carbon chain terminating in a carboxylic acid. It is an oily liquid with an unpleasant, rancid odor. It contributes to the odor of some rancid oils. It is slightly soluble in water, but very soluble in ethanol and ether.??? ??
Heptanoic acid has a disagreeable rancid odor. The spectroscopically pure acid exhibits a faint tallow-like odor. Heptanoic acid may be prepared by oxidation of heptaldehyde with potassium permanganate in diluted sulfuric acid.??
Reported as occurring naturally in calamus, hops, Acacia dealbata, and Japanese peppermint and violet leaves; its presence in rancid oils has been observed Also reported found in passion fruit, mandarin orange peel oil, guava, apple, banana, grapes, papaya, raspberry, strawberry, kiwi, baked potato, sauerkraut, tomato, breads, cheeses, butter, milk, fsh, fsh oil, meats, chicken fat, pork fat, hop oil, beer, cognac, brandy, rum, grape wines, sherry, whiskies, sake, peated malt, cocoa, coffee, tea, soy protein, peanuts, pecans, coconut, beans, mushroom, fenugreek, mango, fgs, licorice, corn oil, shrimps, scallops and other sources??
There are two major uses for heptanoic acid. One is in vinyl plasticizers that are used primarily in the automotive market.This market is expected to grow 3 to 4% per year with GNP.The second is in synthetic lubricants, where heptanoic acid is used in polyol esters.The market for polyol esters is primarily for use in commercial and military jet turbine lubricants.There is a small market for these esters in the automotive lubricant area, but there has been limited acceptance of these products by automakers and the public.The growth of the polyol ester market is expected to track GNP, unless automakers change to support synthetics or unless there is an elevation of military activity.The use of heptanoic acid in high-water metalworking fluids has grown in excess of 20% over the last several years.The amount of acid used in these products is small; therefore, a dramatic change from the traditional oil-based fluids would be required before there would be significant market impact.
?? ??
By oxidation of heptaldehyde with potassium permanganate in diluted sulfuric acid.?? ??
The methyl ester of ricinoleic acid, obtained from castor bean oil is the main commercial precursor to heptanoic acid. It is hydrolyzed to the methyl ester of [[undecenoic acid]] and heptanal, which is then air oxidized to the carboxylic acid. Approximately 20,000 tons were consumed in Europe and US in 1980.Ricinoleic acid is the main precursor to heptanoic acid. Heptanoic acid is used in the preparation of esters, such as ethyl heptanoate, which are used in fragrances and as artificial flavors. Heptanoic acid is used to esterify steroids in the preparation of drugs such as as testosterone enanthate, trenbolone enanthate, drostanolone enanthate and methenolone enanthate (Primobolan). It is also one of many additives in cigarettes.
??
ChEBI: A C7, straight-chain fatty acid that contributes to the odour of some rancid oils. Used in the preparation of esters for the fragrance industry, and as an additive in cigarettes.?? ??
A colorless liquid with a pungent odor. Less dense than water and poorly soluble in water. Hence floats on water. Very corrosive. Contact may likely burn skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Flash point near 200°F.??? ?? ??
Slightly soluble in water.?? ???
Heptanoic acid reacts exothermically with bases. Can react, particularly if moist, with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow if the acid remains dry. Corrodes or dissolves iron, steel, and aluminum parts and containers under ordinary conditions. Reacts with cyanide salts to generate gaseous hydrogen cyanide, particuarly if moist. May generate flammable and/or toxic gases with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Reacts with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reacts exothermically with carbonates and bicarbonates to generate a harmless gas (carbon dioxide). Can be oxidized exothermically by strong oxidizing agents and reduced exothermically by strong reducing agents. A wide variety of products is possible. May initiate polymerization reactions; may catalyze chemical reactions.???
Combustible.????
Harmful if swallowed, inhaled, or absorbed through skin. Extremely destructive to mucous membranes, upper respiratory tract, skin, and eyes. Inhalation may be fatal as a result of spasm, inflammation and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting.????
Heptanoic acid is probably combustible.??? ? ?? ?? ? ???
???
?? ??
??? ? ?? ??
???( 470)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 |
abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12809 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5868 | 58 |
| Hi-Tech Chemistry Corp | 0519-86626038 |
yuhh@hitechem.com | CHINA | 810 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29858 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
??? ? ?? ??:
???? ?? ???? ????? 3,3-???? ???? ? ???? ????????????? ?? ?????? ?? ?????? ????????? ?????????? ??? ?, ?? ???? ,?? ???? ?, ?????? ?, ???? ? AND ??????? ?????????, ???, ???? ???????? ??? ??????, ??? ???? ?????, ????? ? ??????? ?????? ??? ??? ?
Hyaluronic acid
Testosterone enanthate
Methenolone enanthate
1-Heptanol