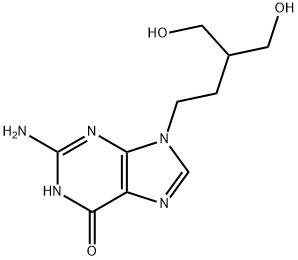
Penciclovir
|
|
Penciclovir ??
- ???
- 275-277°C
- ?? ?
- 653.4±65.0 °C(Predicted)
- ??
- 1.68±0.1 g/cm3(Predicted)
- ?? ??
- Keep in dark place,Sealed in dry,Store in freezer, under -20°C
- ???
- 0.02M ????: ???2mg/mL
- ?? ?? (pKa)
- 14.42±0.10(Predicted)
- ??? ??
- ?? ??
- ??
- ???? ?????
- ???
- water: 1mg/mL
aqueous buffer, pH 2.0: 10mg/mL
- ?? ??(λmax)
- 253nm(H2O)(lit.)
- Merck
- 14,7083
- CAS ??????
- 39809-25-1(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 36 | ||
| ????? | 26 | ||
| WGK ?? | 3 | ||
| RTECS ?? | UP0789400 | ||
| HS ?? | 2933.99.8290 | ||
| ?? ?? ??? | 39809-25-1(Hazardous Substances Data) |
Penciclovir C??? ??, ??, ??
??
Vectavir was launched in the UK for herpes labialis. Penciclovir is synthetically available by two routes of four steps each from 2- (hydroxymethyl)butane-l,4-diol and is active against HSV-1, HSV-2 VZV but has limited activity against CMV. Vectavir is an acyclic guanosine analog that acts as a competitive inhibitor of DNA polymerase. It is a metabolic product of famcyclovir that is preferentially phosphorylated by viral infected cells (by thymidine kinases) over normal cells. The triphosphate has a low activity against cellular DNA polymerase which is one possible explanation for its low toxicity. While its spectrum of activity is similar to acyclovir, it is longer acting because its triphosphate is 20 times more stable and is not metabolized.??? ??
White Cyrstalline Solid??
A deuterated version of Penciclovir, an antiviralIndications
Penciclovir has activity against HSV-1, HSV-2, VZV, and HBV. After oral administration, famciclovir is converted to penciclovir by first-pass metabolism. Penciclovir has a mechanism of action similar to that of acyclovir. It is first monophosphorylated by viral thymidine kinase; then it is converted to a triphosphate by cellular kinases.??
ChEBI: A member of the class of 2-aminopurines that is guanine in which the hydrogen at position 9 is substituted by a 4-hydroxy-3-(hydroxymethyl)but-1-yl group. An antiviral drug, it is administered topically for treatment of herpes labialis. A prodrug, famciclo ir, is used for oral administration.??
Penciclovir is inactive against thymidine kinase-deficient strains of HSV.Pharmaceutical Applications
A synthetic acyclic purine nucleoside analog, usually administered orally as the diacetyl ester, famciclovir, which acts as a prodrug undergoing rapid first-pass metabolism to release the active compound in vivo. The parent compound has virtually no oral bioavailability, but is supplied as a topical formulation.Pharmacokinetics
Oral absorption, penciclovir: 5%famciclovir: 77%
Cmax famciclovir 250 mg oral: 1.6 mg/L after 0.5–1.5 h
famciclovir 500 mg oral: 3.3 mg/L after 0.5–1.5 h
famciclovir 750 mg oral: 5.1 mg/L after 0.5–1.5 h
Plasma half-life: 2.1–2.7 h
Volume of distribution: c. 1.5 L/kg
Plasma protein binding: <20%
Following absorption famciclovir is converted rapidly by enzyme-mediated deacetylation and oxidation to penciclovir. Food does not lead to any significant change in the availability or elimination.
The pharmacokinetics in elderly subjects are similar to those seen in younger subjects, although small increases in AUC and plasma half-lives were seen, consistent with slightly decreased renal clearance.
Renal excretion is the major route of elimination, 50–60% of an oral dose being recovered in the urine. After intravenous infusion, about 70% is excreted unchanged in the urine. After oral administration of famciclovir, penciclovir accounts for 82% of urinary drug-related material. The remainder includes metabolites, of which the largest is the 6-deoxy precursor of penciclovir. Renal clearance exceeds glomerular filtration, indicating renal tubular secretion.
Clinical Use
Penciclovir is approved as a topical formulation for the treatment of herpes labialis. In immunocompetent individuals, penciclovir shortens the duration of lesion presence and pain by approximately half a day when it is initiated within an hour of lesion development and applied every 2 hours during waking hours for 4 days.???
In clinical trials the incidence of adverse events after famciclovir, aciclovir and placebo were similar, the most common adverse events being headache and nausea.Penciclovir ?? ?? ? ???
???
6-??????
???????
??????
?????????
??
??? ?????
p-??? ??? ????
9H-Purin-2-amine, 6-chloro-9-[2-(2,2-dimethyl-1,3-dioxan-5-yl)ethyl]-
5-(2-?????)-2,2-???-1,3-???
2-(2,2-Dimethyl-1,3-dioxan-5-yl)ethanol
2-(??????)??-1,4-??
9-(4-Acetoxy-3-acetoxymethylbutyl)-2-amino-6-chloropurine
1,3-Propanediol, 2-[2-(2-amino-6-chloro-9H-purin-9-yl)ethyl]-
Triethyl 1,1,2-ethanetricarboxylate
?? ??
Penciclovir ?? ??
???( 373)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12818 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5876 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 |
sarah@tnjone.com | China | 1143 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21631 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ |
sales03@shyrchem.com | CHINA | 738 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29863 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 |
faith@widelychemical.com | CHINA | 742 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
Penciclovir ?? ??:
Famciclovir N7-Isomer
9-(2'-ACETOXYETHOXYMETHYL)-GUANINE
N-Methyl Valganciclovir Hydrochloride
Acyclovir IMpurity C
N-t-Boc-valacyclovir
2-[2-(2-AMINO-9H-PURIN-9-YL)ETHYL]-1,3-PROPANEDIOL
Valganciclovir
Valaciclovir EP Impurity H
Entecavir hydrate
Valacyclovir hydrochloride
Vidarabine
Famciclovir
Valganciclovir hydrochloride
FAMCICLOVIR-D4
Famciclovir Related Compound A (20 mg) (2-[2-(2-Amino-9H-purin-9-yl)ethyl]propane-1,3-diol hydrochloride)
MONO-DESACETYL FAMCICLOVIR
FaMciclovir Related CoMpound C
FaMciclovir iMpurity 5