????
|
|
???? ??
- ???
- 210-215 °C (lit.)
- ?? ?
- 340 °C (lit.)
- ??
- 1.28
- ?? ??
- 330kg/m3
- ?? ??
- 6.15 (vs air)
- ???
- 1 mm Hg ( 145 °C)
- ???
- 1.5948
- ???
- 121 °C
- ?? ??
- 2-8°C
- ???
- ???: ???20mg/mL, ??, ?? ?? ??? ???
- ?? ?? (pKa)
- >15 (Christensen et al., 1975)
- ??? ??
- ??
- ?? ?? ??
- 10790
- ??
- ????? ?????
- ????
- 0.6%(V)
- ???
- 0.045mg/L(25℃)
- Merck
- 14,682
- BRN
- 1905429
- Henry's Law Constant
- 1.22 at 4 °C, 6.42 at 25 °C (dynamic equilibrium method, Bamford et al., 1999)
- ?? ??
- OSHA: TWA 0.2 mg/m3
- Dielectric constant
- 2.3500000000000001
- LogP
- 4.65 at 20℃
- CAS ??????
- 120-12-7(CAS DataBase Reference)
- IARC
- 3 (Vol. 92, Sup 7) 2010
- NIST
- Anthracene(120-12-7)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi,N,F,T,Xn | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38-50/53-67-36-11-39/23/24/25-23/24/25-65-38-66-51/53 | ||
| ????? | 26-60-61-24/25-16-9-45-36/37-62-36 | ||
| ????(UN No.) | UN 3077 9/PG 3 | ||
| WGK ?? | 2 | ||
| RTECS ?? | CA9350000 | ||
| ?? ?? ?? | 540 °C | ||
| TSCA | Yes | ||
| ?? ?? | 9 | ||
| ???? | III | ||
| HS ?? | 29029010 | ||
| ?? ?? ??? | 120-12-7(Hazardous Substances Data) | ||
| ?? | LD50 orally in Rabbit: > 16000 mg/kg | ||
| ???? ?? | KE-01825 |
???? C??? ??, ??, ??
??
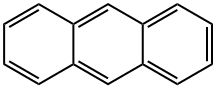
??? ??? ?????.?? ?? 3?? ??? ??? 14?? π??? ?? ?? ??? ????? ??.??
?????? ?? ? ??? π???? ???? ???? ??? ??? ??? ?? ????. ????? ????, ???? ??? ??? ??? ?? ??? ??? ???????? ??? ??? ???? ????.??
Anthracene is one of a group of chemicals called polycyclic aromatic hydrocarbons (PAHs). PAHs are often found together in groups of two ormore. They can exist inmore than 100 different combinations, but the most common are treated as a group of 15. PAHs are found naturally in the environment but they can also be made synthetically. Anthracene can vary in appearance from a colorless to pale yellow crystal-like solid. PAHs are created when products like coal, oil, gas, and garbage are burned but the burning process is not complete. Very little information is available on the individual chemicals within the PAH group; the majority of the information is for the entire PAH group. Anthracene is a solid white to yellow crystal, has a weak aromatic odor, and sinks in water. Its characteristics are boiling point, 3421°C; melting point, 2181°C; molecular weight, 178.22; density/specific gravity, 1.25 at 27 and 41°C; octanol–water coefficient, 4.45. It is soluble in absolute alcohol and organic solvents. Maximum absorption occurs at 218 nm.??? ??
Anthracene is colorless, to pale yellow crystalline solid with a bluish fluorescence. PAHs are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons.??? ??
White to yellow crystalline flakes or crystals with a bluish or violet fluorescence and a weak aromatic odor. Impurities (naphthacene, tetracene) impart a yellowish color with green fluorescence.??
Anthracene is an aromatic hydrocarbonwith three fused rings, and is obtained by the distillationof crude oils. The main useis in the manufacture of dyes.It is an important source of dyestuffs.??
(C14H10) A white crystalline solid used extensively in the manufacture of dyes. Anthracene is found in the heavy- and green-oil fractions of crude oil and is obtained by fractional crystallization. Its structure is benzene-like, having three six-membered rings fused toanion gether. The reactions are characteristic of AROMATIC COMPOUNDS.?? ??
Anthracene is obtained from coal tar in the fraction distilling between 300° and 400 °C. This fraction contains 5–10% anthracene, from which, by fractional crystallization followed by crystallization from solvents, such as oleic acid, and washing with such solvents as pyridine, relatively pure anthracene is obtained. It may be detected by the formation of a blue-violet coloration on fusion with mellitic acid. Anthracene derivatives, especially anthraquinone, are important in dye chemistry.?? ??
Anthracene reacts: (1) With oxidizing agents, e.g., sodium dichromate plus sulfuric acid, to form anthraquinone, C6H4(CO)2C6H. (2) With chlorine in water or in dilute acetic acid below 250 °C to form anthraquinol and anthraquinone, at higher temperatures 9,10-dichloroanthracene. The reaction varies with the temperature and with the solvent used. The reaction has been studied using, as solvent, benzene, chloroform, alcohol, carbon disulfide, ether, glacial acetic acid, and also without solvent by heating. Bromine reacts similarly to chlorine. (3) With concentrated sulfuric acid to form various anthracene sulfonic acids. (4) With nitric acid, to form nitroanthracenes and anthraquinone. (5) With picric acid (1)HO·C6H2(NO2)3(2,4,6) to form red crystalline anthracene picrate, melting point 138 °C.?? ??
White to yellow solid with a weak aromatic odor. Sinks in water.??? ?? ??
Flammable. Insoluble in water.?? ???
Anthracene will spontaneously burst into flame on contact with chromic acid, and other strong oxidants.???
A questionable carcinogen.????
Carcinogenicity of anthracene is not known.Its toxicity is very low. An intraperitonealLD50 in mice is recorded at 430 mg/kg(NIOSH 1986).????
Anthracene is combustible.Safety Profile
Moderately toxic by intraperitoneal route. A skin irritant and allergen. Questionable carcinogen with experimental neoplas tigenic and tumorigenic data. Mutation data reported. Combustible when exposed to heat, flame, or oxidizing materials. Moderately explosive when exposed to flame, Ca(OCl)z, chromic acid. To fight fire, use water, foam, CO2, water spray or mist, dry chemical. Explodes on contact with fluorine.??? ??
It is used as an intermediate in dye stuffs (alizarin), insecticides, and wood preservatives; making synthetic fibers, anthraquinone, and other chemicals. May be present in coke oven emissions, diesel fuel, and coal tar pitch volitiles.Carcinogenicity
Anthracene was negative in mouse-skin-painting studies, and it is classified as a noncarcinogen by the IARC based on inadequate evidence. The methyl, anthryl, dimethyl, diprophyl, dinaphthyl, and tetramethyl derivatives of anthracene were noncarcinogenic except for 9,10-dimethyl anthracene, which may have contained impurities when tested.?? ??
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.? ???
Finely dispersed powder may form explosive mixture in air. Contact with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, chromic acid/or calcium hypochlorite.??? ??
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration.???? ?? ?? ? ???
???
UP? ??? ???
?? ??
????
Anthracene oil
??? ???
????
Tetracyclo[6.6.2.02,7.09,14]hexadecane-2(7),3,5,9(14),10,12-hexene-15,15,16,16-tetracarbonitrile
9,9'-Spirobi[9H-xanthene]
?? ??
Vat Brown 3GR
Early-strength admixture
1-NITROANTHRAQUINONE
Pumping agent
????
???? -9- ?? ? ????
?????? ???????
?????
Maprotiline
9,10-Dibromoanthracene
9,10-Bis(chloromethyl)anthracene
9,10-?????????????
Slushing agent,high efficiency
????
9-??????
1,5-DIHYDROXYANTHRAQUINONE
????,??????????
Sulfurized anthracene
???? ?? ??
???( 392)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Mascot I.E.Co .,Ltd. | +86-0519-85010339 +8613584504415 |
info@mascotchem.com | China | 327 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20235 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-+86-371-66670886 |
info@dakenam.com | China | 19805 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1803 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1001 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29858 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |