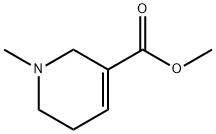
ChemicalBook >?? ???? >????
????
|
|
???? ??
- ???
- <25℃
- ?? ?
- 209℃
- ??
- 1.0504 g/cm3 (20 ºC)
- ???
- 1.4860 (589.3 nm 20℃)
- ?? ??
- Sealed in dry,Room Temperature
- ???
- ?????(???? ??), ???(?? ???)
- ??? ??
- ??
- ?? ?? (pKa)
- 6.84(at 25℃)
- ??
- ??? ??
- LogP
- 0.350
??
| ?? | LD50 in mice, dogs (mg/kg): 100, 5 s.c. (Burrows) |
|---|
???? C??? ??, ??, ??
??
Areca catechu L. (Bin Lang), a palm plant native to Malaysia, is mainly distributed in tropical regions of Asia and the Americas. Arecoline is extracted from dry mature seeds of areca. The mature seeds of areca are 3–5 cm in diameter, with fibrous peel and a seed, namely, areca nut. The endosperm of areca nut is hard, with grayish-brown spots. Areca nut is harvested from August to November every year before the fruit is fully mature. The seeds are peeled, boiled, and cut into thin slices. The dried slices are brown or black, and it is an important medicine in traditional Chinese medicine. Areca nut is the raw materials of catechu. Areca nut was used as anthelmintics with arecoline as the main alkaloid in it.??? ??
Oily Liquid??? ??
Appearance: oily liquid. Solubility: mixed with water, ethanol, or ether in any ratio, soluble in chloroform. Density: 1.059?g/cm3 . Boiling point (760?mmHg): 209?°C. Flash point: 81.1?°C. Vapor pressure (25?°C): 0.208?mmHg. Arecoline can be synthesized to salts with organic acids. Arecoline hydrobromide, arecoline acetarsol, and arecoline p-antimony carboxybenzoic acid are commonly used.??
Arecoline, an alkaloid extracted from Areca catechu L., is first reported by Jahns in 1888. Mujumdar found that there were at least six kinds of alkaloids in areca, including arecoline, arecaidine, guavacoline, and guavacine. The content of arecoline in the fresh fruit of areca was 0.3–0.63% as determined with HPLC .It has been found first that arecoline has the effect of antiparasite and is promoting the motility of the gastrointestinal smooth muscle. It activates the M and N cholinergic receptors, excites the nervous system, promotes the body excitability, and improves the ability of learning and memory .
??
A cholinergic alkaloid from seeds of the betel nut palm Areca catechu. Anthelmintic (Cestodes); catharticIndications
This product has no source standard. Tablets: anthelmintics, less used now. Eye drops: cholinergic drugs for glaucoma treatment.??
ChEBI: A tetrahydropyridine that is 1,2,5,6-tetrahydropyridine with a methyl group at position 1, and a methoxycarbonyl group at position 3. An alkaloid found in the areca nut, it acts as an agonist of muscarinic acetylcholine.Pharmacology
Paralysis was the main mechanism of the antiparasite effect of arecoline. The effect of arecoline on cholinergic receptors is similar to that of pilocarpine. It can agonize M-cholinergic receptors (M1, M2, M3, M4) and increase the secretion of glands, especially the salivary. It can also agonize N-cholinergic receptors and activate the muscles of skeletal, ganglia, carotid body, etc. The central nervous system is also influenced by the cholinergic effect of arecoline. Intravenous injection of small dosage of arecoline can cause wake-up reaction of the cortex in cats, which is inhibited or blocked by atropine. Arecoline can cause salivation, vomiting, diuretic, lethargy, and convulsion when overdosed.In addition, arecoline can enhance intestinal peristalsis, contract bronchus, lower heart rate, dilate blood vessels, and decrease blood pressure. While in rabbit, it induces coronary artery contraction.
Eye drops can reduce the pupil.
Pharmacokinetic parameters for arecoline after oral administration of arecoline (3? mg/kg): Tmax, 120.07? min; Cmax, 60.61? ng/mL; t1/2, 69.32? min; AUC0? – t, 15116.86? min/ng/mL; AUC0-∞, 15771.37? min/ng/mL; plasma clearance, 0.19? L/ min/kg .
Clinical Use
Arecoline is of historical interest, because its structure, like those of many other early medicinal agents, was determined and confirmed by a 19th-century German pharmacist, E. Jahns. Xanomeline may be viewed as a nonclassical bio-isostere of the ester moiety of arecoline. It is a muscarinic M1/M4 agonist that is showing promise in clinical trials for the treatment of Alzheimer's disease. Although it is not tolerated at orally effective doses, transdermal delivery systems are showing promise.Safety Profile
Poison by subcutaneous and intraperitoneal routes. Moderately toxic by ingestion. Questionable carcinogen with experimental neoplastigenic data. It mimics the action of acetylcholine, a neurotransmitter, and is a parasympathetic nervous system stimulant. Its action on the central nervous system can cause tremors. Human mutation data reported. It is easily nitrosated to several nitrosamines. See also ESTERS and NITROSAMINES. It is the major alkaloid found in betel quid. Combustible, can react with oxidzing materials. When heated to decomposition it emits hghly toxic fumes of NOx.???? ?? ?? ? ???
???
?? ??
???? ?? ??
???( 103)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +8618829768577 |
gao490372054@gamil.com | China | 1249 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 997 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 |
ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| Wuhan ChemNorm Biotech Co.,Ltd. | +86-27-8439 4403 18971486879 |
sales@chemnorm.com | CHINA | 2935 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32159 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 |
laboratory@coreychem.com | China | 30231 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7722 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
sales@afinechem.com | China | 15352 | 58 |
???? ?? ??:
????
METHYL 4-AMINO-1-BENZYL-1,2,5,6-TETRAHYDROPYRIDINE-3-CARBOXYLATE, 99
ARECAIDINE BUT-2-YNYL ESTER TOSYLATE
ARECOLINE HYDROCHLORIDE
ARECAIDINE PROPARGYL ESTER TOSYLATE
ECGONIDINE METHYL ESTER MESYLATE
CHEMBRDG-BB 6668327
arecoline
ethyl 1-benzyl-1,2,5,6-tetrahydropyridine-3-carboxylate
ARECAIDINE PROPARGYL ESTER HYDROBROMIDE (APE)
N-BENZYL-3-CARBOMETHOXY-4-PIPERIDONE, SODIUM SALT MONOHYDRATE, TECH.
CBI-BB ZERO/005862
RARECHEM AX KI 5013
RARECHEM AX KI 5016
arecoline methiodide
RARECHEM AX KI 5014
RARECHEM AX KI 5015
RARECHEM AX KI 5017