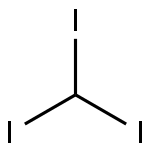
?????
|
|
????? ??
- ???
- 119 °C
- ?? ?
- 218°C
- ??
- 4.008 g/mL at 25 °C(lit.)
- ???
- 1.4975 (estimate)
- ?? ??
- -20°C
- ???
- ?????: ???5%
- ??? ??
- ??? ??
- ??
- ?? ????? ??? ?????
- ??
- ?? ??. ???? ??
- ???? ??
- mouse
- ???
- ???. 24&C?? <0.1g/100mL
- ??
- Light Sensitive
- Merck
- 14,5033
- BRN
- 1697010
- ?? ??
- ACGIH: TWA 0.6 ppm
NIOSH: TWA 0.6 ppm(10 mg/m3)
- ???
- ????. ?? ???, ???? ???? ????. ???? ??? ? ??.
- LogP
- 3.510 (est)
- CAS ??????
- 75-47-8(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xn | ||
|---|---|---|---|
| ?? ???? ?? | 20/21/22-36/37/38 | ||
| ????? | 26-36/37-36/37/39-22-24/25 | ||
| ????(UN No.) | UN 1851 | ||
| OEB | B | ||
| OEL | TWA: 0.6 ppm (10 mg/m3) | ||
| WGK ?? | 3 | ||
| RTECS ?? | PB7000000 | ||
| F ?????? | 13 | ||
| TSCA | Yes | ||
| HS ?? | 29033990 | ||
| ?? ?? ??? | 75-47-8(Hazardous Substances Data) | ||
| ?? | LD50 s.c. in mice: 1.6 mmoles/kg (Kutob, Plaa) | ||
| ???? ?? | KE-34308 |
????? C??? ??, ??, ??
??
Iodoform is a stable, pale yellow crystalline solid. It is volatile with a characteristic pungent, unpleasant and penetrating odour, but with a sweetish taste. It is incompatible with strong oxidising agents, reducing agents, lithium, and metallic salts such as mercuric oxide, silver nitrate, strong bases, calomel, and tannin. Reports indicate that earlier iodoform was in use as a disinfectant and as an antiseptic or dressing and healing of wounds and sores for pets. Iodoform is the active ingredient in many antiseptic or dressing powders used for dogs and cats to prevent infection.??? ??
Iodoform is a yellow or greenish-yellow powder or crystalline solid that is volatile with steam and contains 96.69% iodine. It has a very characteristic pungent odor. An odor threshold of 0.005 ppm has been reported.??
Iodoform has limited use as a chemical intermediate and for medicinal purposes as disinfectant and antiseptic and has been used in veterinary medicine.??
A yellow crystalline compound made by warming ethanal with an alkaline solution of an iodide:CH3CHO + 3I– + 4OH– → CHI3 +
HCOO– + 3H2O
The reaction also occurs with all ketones of general formula CH3COR (R is an alkyl group) and with secondary alcohols CH3CH(OH)R. Iodoform is used as a test for such reactions (the iodoform reaction).
?? ??
Bright yellow or yellow powder or crystals. Penetrating odor. Unctuous touch. Odor threshold 0.4 ppb.??? ?? ??
Insoluble in water.?? ???
Iodoform decomposes at high temperatures. Decomposes slowly in light at room temperature. Reacts violently with lithium. Is incompatible with mercuric oxide, calomel, silver nitrate, tannin, and balsam Peru. Is also incompatible with strong bases, strong oxidizing agents and magnesium. Vigorous reactions occur with acetone in the presence of solid potassium hydroxide or calcium hydroxide, hexamethylenetetramine at 352° F, mercury(I) fluoride and finely divided reduced silver.???
Irritant. Decomposes violently at 400F (204C).????
Iodoform causes central nervous system depression and damage to the kidneys, liver, and heart.????
Literature sources indicate that Iodoform is nonflammable.Carcinogenicity
Iodoform has been included in the NCI daily bioassay program. According to their summary: the high and low time-weighted average daily dosages of iodoform were, respectively, 142 and 71 mg/kg for male rats, 55 and 27 mg/kg for female rats, and 93 and 47 mg/kg for male and female mice. A significant positive association between dosage and mortality was observed in male rats but not in female rats or in mice of either sex. Adequate numbers of animals in all groups survived sufficiently long to be at risk from late-developing tumors. No statistical significance could be attributed to the incidences of any neoplasms in rats or mice of either sex when compared to their respective controls. Under the conditions of this bioassay, no convincing evidence was provided for the carcinogenicity of iodoform in Osborne–Mendel rats or B6C3F1 mice.Purification Methods
Crystallise it from MeOH, EtOH or EtOH/EtOAc. It is steam volatile. It is a disinfectant. [Beilstein 1 IV 97.]????? ?? ?? ? ???
???
?? ??
????? ?? ??
???( 300)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5876 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21631 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 |
info@longyupharma.com | China | 2555 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29808 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9636 | 58 |
????? ?? ??:
??? ?? ???????? ????????? ???????? ?? ?????
TRIIODOACETIC ACID
IODOFORM-D 99 ATOM % D,IODOFORM-D
Methyl iodoform
PROTEOGEL IPG STRIP 7 CM PH 6-11
PROTEOGEL IPG STRIP 18 CM PH 3-5
PROTEOGEL IPG STRIP 18 CM PH 8-11
PROTEOGEL IPG STRIP 11 CM PH 3-10
PROTEOGEL IPG STRIP 11 CM PH 3-5
PROTEOGEL IPG STRIP 18 CM PH 6-11
PROTEOGEL IPG STRIP 7 CM PH 3-5
PROTEOGEL IPG STRIP 11 CM PH 8-11
PROTEOGEL IPG STRIP 7 CM PH 8-11
PROTEOGEL IPG STRIP 18 CM PH 5-8