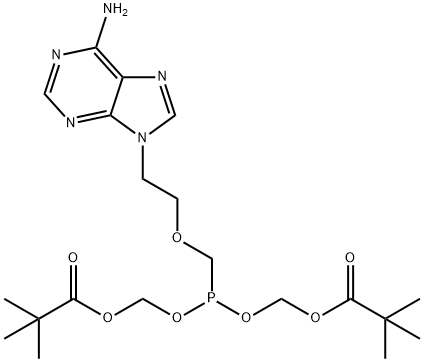
Adefovir dipivoxil
|
|
Adefovir dipivoxil ??
- ???
- 98-102°C
- ?? ?
- 641.0±65.0 °C(Predicted)
- ??
- 1.35±0.1 g/cm3(Predicted)
- ?? ??
- Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
- ???
- ???: ???50mg/mL
- ?? ?? (pKa)
- 4.16±0.10(Predicted)
- ??? ??
- ??
- ??? ??
- ??? ??
- ??
- ???? ?????
- ???? ??
- synthetic
- Merck
- 14,151
- InChIKey
- WOZSCQDILHKSGG-UHFFFAOYSA-N
- CAS ??????
- 142340-99-6(CAS DataBase Reference)
??
- ?? ? ?? ??
Adefovir dipivoxil C??? ??, ??, ??
??
Adefovir dipivoxil is the first nucleotide analog to be launched in the US as an oral treatment for hepatitis B virus (HBV) infections. It can be easily prepared in 4 steps from adenine. Adefovir dipivoxil acts as a bioavailable ester prodrug which is rapidly hydrolyzed to free adefovir and further anabolized to its active form, adefovir diphosphate, by two intracellular phosphotylation steps. The diphosphate competitively inhibits reverse transcriptase and/or causes chain termination when incorporated into growing DNA. Adefovir dipivoxil has a broad antiviral spectrum against retro-, herpes- and hepadnaviruses. The drug inhibits HBV replication, decreases HBV DNA levels and improves liver histology of patients infected with HBV wild type and resistant to other antivirals such as lamivudine. It also demonstrated activity in hepatitis B”e” antigennegative, or precore mutant, patients and in patients co-infected with HIV. To date, no adefovir dipivoxil-associated resistance mutations have been identified in patients up to 136 weeks with the drug. The oral bioavailability of adefovir after oral administration of its dipivoxil prodrug is approximately 30%. It is mainly excreted unchanged in the urine and its plasma elimination half-life is 4.2 h. However, a long intracellular half-life (17 h) of the active bisphosphorylated metabolite enables once-daily dosing. The most prominent adverse effect of adefovir dipivoxil is nephrotoxicity (which has prevented the drug from being marketed for HIV infections where the drug required administration at higher doses).??? ??
White Solid??
Adefovir Dipivoxil(Preveon, Hepsera) works by blocking reverse transcriptase, an enzyme that is crucial for the hepatitis B virus (HBV) to reproduce in the body. It is approved for the treatment of chronic hepatitis B in adults with evidence of active vir?? ??
Adefovir is an orally active prodrug that is indicated for thetreatment of the chronic form of hepatitis B. The dipivoxil moieties are hydrolyzed by ubiquitous esterases to yieldadefovir, which is phosphorylated by adenylate kinase toyield adefovir diphosphate. This compound is inhibitory atHBV DNA polymerase. In addition, adefovir undergoes incorporationinto viral DNA and causes chain termination.Adefovir is poorly absorbed by the oral route, but the dipivoxilester groups cause the bioavailability to increase toapproximately 60%.Mechanism of action
Adefovir dipivoxil is an orally active prodrug indicated for the treatment of chronic hepatitis B. The drug is hydrolyzed by extracellular esterases to produce adefovir, which in turn is phosphorylated by adenylate kinase to adefovir diphosphate, which inhibits HBV DNA polymerase. Incorporation of adefovir into viral DNA also leads to DNA chain termination. As shown in Figure 43.9, adefovir dipivoxyl is activated in two steps involving an esterase that exposes a free phosphate group (adefovir), followed by addition of a second phosphate by adenylate kinase to form adefovir diphosphate, the active form of the drug.Clinical Use
Adefovir dipivoxil joins interferon and lamivudine in the treatment of chronic HBV. It can be used singly or in combination with lamivudine. Early clinical studies indicate benefit of the use of adefovir dipivoxil to treat lamivudine-resistant HBV with a low level of resistant virus developing to monotherapy with adefovir dipivoxil.?? ??
Adefovir is poorly absorbed orally, but the bioavailability of adefovir dipivoxil reaches approximately 59%. The drug is absorbed to an equal extent with or without the presence of food. Adefovir is excreted renally unchanged.Adefovir dipivoxil ?? ?? ? ???
???
??? ??????
????
9H-Purine-9-acetic acid, 6-chloro-, ethyl ester
2-(6-???-??-9-?)-???
Iodomethyl pivalate
Adefovir
[[2-(6-???-9H-??-9-?)???]??]???????????
2-(6-?????-9-?)???
?????-2,2-??????????
???
?? ??
Adefovir dipivoxil ?? ??
???( 564)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 |
sarah@tnjone.com | China | 1143 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +8613288715578 |
angelia@hbmojin.com | China | 1174 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29792 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ |
sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32965 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29871 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 |
sales@czwytech.com | CHINA | 904 | 58 |