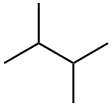
2,3-?????
|
|
2,3-????? ??
- ???
- -129 °C
- ?? ?
- 58 °C(lit.)
- ??
- 0.662 g/mL at 25 °C(lit.)
- ?? ??
- 3 (vs air)
- ???
- 7.41 psi ( 37.7 °C)
- ???
- n
20/D 1.375(lit.)
- ???
- −28 °F
- ?? ??
- Flammables area
- ???
- In methanol: 495, 593, 760, and 1,700 g/L at 5, 10, 15, and 20 °C, respectively. Miscible at higher temperatures (Kiser et al., 1961).
- ?? ?? (pKa)
- >14 (Schwarzenbach et al., 1993)
- ??? ??
- ??
- ??
- ???
- ????
- ~7.7%
- Odor Threshold
- 0.42ppm
- ???
- ?(22°C?? 1mg/ml), DMSO, ???, ???? ?????.
- BRN
- 1730737
- Henry's Law Constant
- 1.28(atm?m3/mol) at 25 °C (Mackay and Shiu, 1981)
- ?? ??
- ACGIH TLV: TWA and STEL for all isomers except n-hexane are 500 and 1,000 ppm, respectively (adopted).
- Dielectric constant
- 1.8899999999999999
- ???
- ????. ???. ??? ?? ??? ???? ?????. ?? ???? ?????. ?? ???, ??? ???? ????.
- InChIKey
- ZFFMLCVRJBZUDZ-UHFFFAOYSA-N
- LogP
- 3.42
- CAS ??????
- 79-29-8(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | F,Xn,N | ||
|---|---|---|---|
| ?? ???? ?? | 11-65-67-51/53-38 | ||
| ????? | 16-23-33-62-61-29-9 | ||
| ????(UN No.) | UN 2457 3/PG 2 | ||
| OEB | A | ||
| OEL | TWA: 100.0 ppm; 350.0 mg/m3, STEL: 510.0 ppm; 1800.0 mg/m3 | ||
| WGK ?? | 3 | ||
| RTECS ?? | EJ9350000 | ||
| ?? ?? ?? | 788 °F | ||
| TSCA | Yes | ||
| ?? ?? | 3 | ||
| ???? | II | ||
| HS ?? | 29011000 | ||
| ?? ?? ??? | 79-29-8(Hazardous Substances Data) | ||
| ???? ?? | KE-11249 |
2,3-????? C??? ??, ??, ??
??? ??
2,3-Dimethylbutane, C6H14, is a flammable liquid with a specific gravity of 0.66164. It is released into the atmosphere from automobile, biomass combustion, and gasoline vapor emissions.??? ??
Colorless liquid with a mild gasoline-like odor. An odor threshold concentration of 4.2 ppmv was reported by Nagata and Takeuchi (1990).??
2,3-Dimethylbutane is used in high octane fuels and in organic synthesis. It is also used as a gas chromatography standard.?? ??
2,3-Dimethylbutane is produced from crude oil, natural liquid gases, and petroleum refining processes.?? ??
A clear colorless liquid with a petroleum-like odor. Flash point -20°F. Less dense than water and insoluble in water. Vapors heavier than air.??? ?? ??
Highly flammable. Insoluble in water.?? ???
Saturated aliphatic hydrocarbons, such as 2,3-DIMETHYLBUTANE, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. 2,3-DIMETHYLBUTANE is incompatible with oxidizing materials. 2,3-DIMETHYLBUTANE is also incompatible with oxygen. .????
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.Safety Profile
Probably an irritant and narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.????
Photolytic. Major products reported from the photooxidation of 2,3-dimethylbutane with nitrogen oxides are carbon monoxide and acetone. Minor products included formaldehyde, acetaldehyde and peroxyacyl nitrates (Altshuller, 1983). Synthetic air containing gaseous nitrous acid and exposed to artificial sunlight (λ = 300–450 nm) photooxidized 2,3-dimethylbutane into acetone, hexyl nitrate, peroxyacetal nitrate, and a nitro aromatic compound tentatively identified as a propyl nitrate (Cox et al., 1980).Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor. 2,3- Dimethylbutane will not hydrolyze because it has no hydrolyzable functional group.
Purification Methods
Distil it from sodium, pass it through a column of silica gel (activated by heating in nitrogen to 350o before use) to remove unsaturated impurities, and again distil it from sodium. Also distil it azeotropically with MeOH, then wash with water, dry (Na2SO4) it, and redistil it. [Beilstein 1 IV 371.]2,3-????? ?? ?? ? ???
???
?? ??
2,3-????? ?? ??
???( 161)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8803 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20258 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 10453 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29809 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9636 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8670 | 58 |
2,3-????? ?? ??:
2-???-3,3-?????? 3,7,7-??????????[4.1.0]?-3-? ??-?? ?? ??????? (+)-A-?? 3,3-???-2-?? ????? ?????? ????????????? ???? 2-???? 2,2-????? ?????
2,3-Dimethylbutane-2,3-diol,2,3-Dihydroxy-2,3-dimethylbutane
PINONIC ACID
ALPHA-PINENE
ALPHA-(-)-THUJONE
1,1-DICHLORO-3,3-DIMETHYLBUTANE
CYCLOPROPYL DIPHENYL CARBINOL