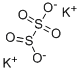?? ???????
|
|
?? ??????? ??
- ???
- 190°C
- ??
- 2.34 g/cm3
- ?? ??
- 1000-1300kg/m3
- ?? ??
- 2.3 (vs air)
- ?? ??
- Store at +5°C to +30°C.
- ???
- H2O: 0.1 M at 20 °C, ??, ??
- ??? ??
- ??/??
- Specific Gravity
- 2.3
- ??
- ???? ?? ???
- ??????(pH)
- 2.5-4.5 (25℃, 0.1M in H2O)
- ??
- ?? ??
- pH ??
- 3-4.5 at 22.2 g/l at 25 °C
- ???
- ?? ?????. ???? ???.
- ??
- Air Sensitive
- ????
- 150°C
- ?? ??(λmax)
- 267nm(H2O)(lit.)
- Merck
- 14,7645
- Absorption
- cut-off at 308nm in H2O at 0.1M
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 31-36/37/38-41 | ||
| ????? | 26-36-39 | ||
| WGK ?? | 1 | ||
| RTECS ?? | TT4920000 | ||
| F ?????? | 23 | ||
| TSCA | Yes | ||
| HS ?? | 28322000 | ||
| ?? ?? ??? | 16731-55-8(Hazardous Substances Data) | ||
| ?? | LD50 orally in Rabbit: 2300 mg/kg | ||
| ???? ?? | KE-12700 |
?? ??????? C??? ??, ??, ??
????
? (1) ?? : ? ?? 1g? ? 10mL? ?? ?, ? ??? ?? ?? ????? ??.
??(2) ?? : ? ??? ?????? ?? ??? ?, ? ?? 4.0ppm ????? ??.
??(3) ? : 「???????」? ???? (2)? ?? ????(2.0ppm ??).
??(4) ? : ???? (3)? ????? ??????? ?? ?????????????? ?? ??? ?, ? ?? 10ppm ????? ??.
??(5) ??? : ? ?? 2.0g? ??? ?? 50mL ???? ?? ? 10mL, ?? 5mL? ?? ??? ????? ??? ?? ?????? ??. ?? ???? ? ?? 1.0g? ??? ?? ?? ??????? 0.5mL? ?? ?? ????? ?? ???? ???? ????? ??. ? ???? ?????? 2g? ?? ???? ?? ??, ????? ??? ?? ??? 50mL? ? ?? ?? ????? ?, ????? ??? ??? ?? ????? ?? ??(5ppm ??).
??(6) ?? : ? ??? ?????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(7) ????? : ? ??? 10% ???? ?? ?? ??? ??? ???? ? ?, ? ?? ????? ??(0.1% ??).
????
? (1) ? ??? ????? ??? ????? ????.
??(2) ? ??? ???(1→10) 5mL? ???? 1mL? ??? ???????????? ???? ??? ?? ????.
??(3) ? ??? ????? ? ???? ??? ????.
???
? ? ?? ? 0.2g? ??? ?? ?? 0.1N ????? 50mL? ?? ??????? ?? ??? ??? ?? 5?? ??? ?? ??(2→3) 2mL? ???. ??? ??? ???? 0.1N ??????????? ????(??? : ????).
0.1N ????? 1mL = 5.558mg K2S2O5
??? ??
Potassium metabisulfite occurs as white or colorless free-flowing crystals, crystalline powder, or granules, usually with an odor of sulfur dioxide.??
Potassium Metabisulfite is a chemical preservative and antioxi- dant obtained as white or colorless crystals, powder, or granules. it is soluble in water and insoluble in alcohol. the sulfite salt yields sulfurous acid at a low ph. it is used as a food preservative.?? ??
Potassium disulfite (Potassium metabisulfite, PMB) is an inorganic salt with antimicrobial properties. It is a sulfiting agent that prevents browning of foods.Pharmaceutical Applications
Potassium metabisulfite is used in applications similar to those of sodium metabisulfite in pharmaceuticals, and in the food, brewing, and wine making industries. It is used as an antioxidant, antimicrobial preservative and sterilizing agent.?? ?? ??
Potassium metabisulfite is an antioxidant used as an antifermentative agent in breweries and wineries, as a preservative of fruits and vegetables, and to bleach straw. Reactions to both sodium and potassium metabisulfite are expected.Safety Profile
Experimental reproductive effects. A very irritating material. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and K2SO3. See also SULFITES.Safety
Potassium metabisulfite is used in a variety of foods and pharmaceutical preparations, including oral, otic, rectal, and parenteral preparations. Potassium metabisulfite is considered a very irritating material, and may cause dermatitis on exposed skin.Hypersensitivity reactions to potassium metabisulfite and other sulfites, mainly used as preservatives in food products, have been reported. Reactions include bronchospasm and anaphylaxis; some deaths have also been reported, especially in those with a history of asthma or atopic allergy.These reactions have led to restrictions by the FDA on the use of sulfites in food applications. However, this restriction has not been extended to their use in pharmaceutical applications. Indeed, epinephrine (adrenaline) injections used to treat severe allergic reactions may contain sulfites.
The WHO has set an acceptable daily intake of sulfites, as SO2, at up to 0.35 mg/kg body-weight.
??
Potassium metabisulfite should be stored in a cool, dark place. When stored at a maximum temperature of 25°C and maximum relative humidity of 45%, the shelf-life is 6 months. Potassium metabisulfite decomposes at temperatures above 150°C. In the air, it oxidizes to the sulfate, more readily in the presence of moisture.In aqueous solution, potassium metabisulfite forms potassium bisulfite (KHSO3) which exerts a strong reducing effect.
? ???
Potassium metabisulfite is incompatible with strong acids, water, and most common metals. It reacts with nitrites and sodium nitrate at room temperature, which occasionally results in the formation of flame. The reaction may be explosive if water is present. Potassium metabisulfite liberates SO2 with acids.Sulfites, including potassium metabisulfite, can react with various pharmaceutical compounds including sympathomimetics such as epinephrine (adrenaline),chloramphenicol,cisplatin, and amino acids,which can result in their pharmacological inactivation. Sulfites are also reported to react with phenylmercuric nitrate,and may adsorb onto rubber closures.
Regulatory Status
GRAS listed. Accepted in Europe for use as a food additive in certain applications. Included in the FDA Inactive Ingredients Database (IM and IV injection; otic and rectal solutions and suspensions). Included in the Canadian List of Acceptable Nonmedicinal Ingredients.?? ??????? ?? ?? ? ???
???
?? ??
?? ??????? ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5855 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18147 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 662 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18951 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29871 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 |
sale04@alfachem.cn | China | 11855 | 58 |