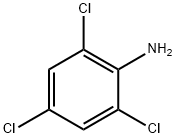
2,4,6-?????????
|
|
2,4,6-????????? ??
- ???
- 73-75 °C (lit.)
- ?? ?
- 262 °C (lit.)
- ??
- 1.6807 (rough estimate)
- ???
- 0-0.631Pa at 25℃
- ???
- 1.6300 (estimate)
- ???
- 262°C
- ?? ??
- Storage temperature: no restrictions.
- ???
- 0.039g/l ?? ???
- ?? ?? (pKa)
- 0.07±0.10(Predicted)
- ??
- ?? ????? ?? ??? ?? ??
- ???
- 21.5&C?? <0.1g/100mL
- BRN
- 879568
- ???
- ?????? ???? ??? ??? ? ????. ?, ? ???, ? ???, ????????, ????? ???? ????.
- InChIKey
- NATVSFWWYVJTAZ-UHFFFAOYSA-N
- LogP
- 3.648-3.69 at 25℃
- CAS ??????
- 634-93-5(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | T,N | ||
|---|---|---|---|
| ?? ???? ?? | 23/24/25-33-50/53 | ||
| ????? | 28-36/37-45-60-61-28A | ||
| ????(UN No.) | UN 2811 6.1/PG 2 | ||
| WGK ?? | 3 | ||
| RTECS ?? | BZ0250000 | ||
| ?? ?? ?? | Toxic | ||
| TSCA | Yes | ||
| ?? ?? | 6.1 | ||
| ???? | II | ||
| HS ?? | 29214200 | ||
| ?? ?? ??? | 634-93-5(Hazardous Substances Data) | ||
| ?? | LD50 orl-rat: 2400 mg/kg GISAAA 55(6),86,90 | ||
| ???? ?? | KE-34061 |
2,4,6-????????? C??? ??, ??, ??
??
Trichloroaniline is particularly important as a chemical intermediate in manufacturing many benzene derivatives and other organic compounds. For instance, 2,4,6-trichloroaniline is an essential precursor of 1,3,5-trichlorobenzene. The latter has been extensively studied and developed for insecticides and fungicides. It, in turn, is a precursor of trichlorodinitrobenzene, which is especially important in combatting crop diseases and is an intermediate for dyestuffs. In military applications, 2,4,6-trichloroaniline is utilized for the preparation of insensitive energetic material that is employed in several Navy weapons systems. The synthesis of 2,4,6-trichloroaniline has almost invariably been accomplished by chlorinating aniline with chlorine or sulfuryl chloride.??? ??
fine purple fibres??
2,4,6-trichloroaniline is important as an intermediate in the manufacture of benzene derivatives, including 1,3,5-trichlorobenzene and is useful in the formation of fungicides, mono-azo dyestuffs, and the preparation of hexachlorodiphenyl urea.Tetrachloronitrobenzene was prepared from 2:4:6-trichloroaniline,which was converted by a Sandmeyer reaction into 1:2:3:5- tetrachlorobenzene.
?? ??
Long needles or fine, light purple fibers.??? ?? ??
2,4,6-Trichloroaniline may be sensitive to exposure to light and air. Insoluble in water.?? ???
2,4,6-Trichloroaniline is incompatible with acids, acid chlorides, acid anhydrides, chloroformates, and strong oxidizing agents. .????
Flash point data for 2,4,6-Trichloroaniline are not available. 2,4,6-Trichloroaniline is probably combustible.Safety Profile
Moderately toxic by ingestion.Irritant. Questionable carcinogen with experimentalcarcinogenic data. Mutation data reported. When heatedto decomposition it emits very toxic fumes of Clí andNOx.Purification Methods
Crystallise the aniline from ligroin. The benzoyl derivative has m 174o (from EtOH). [Beilstein 12 H 627, 12 IV 1281.]2,4,6-????????? ?? ?? ? ???
???
?? ??
2,4,6-????????? ?? ??
???( 254)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Shanghai Worldyang Chemical Co.,Ltd. | +86-021-56795779 +8613651600618 |
sales@worldyachem.com | China | 1073 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5893 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20288 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29791 | 60 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9218 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29886 | 58 |
2,4,6-????????? ?? ??:
P-?????? M-?????? 3,4-??????? 2,5-??????? 2,6-??????? 2,4-??????? 2-?????? 2,4,6-??????? ????????? 2,4,6-??????? ??????? ?????????? 1,3,5-?????-2-????? 2,4,5-???????? 2,3,4-???????? 3,4,5-???????? 2,4,6-?????????
Pentachloroaniline
1-(2,4,6-Trichlorophenyl)-3-amino-pyrazolin-5-one
1-(2,4,6-TRICHLOROPHENYL)-2-THIOUREA