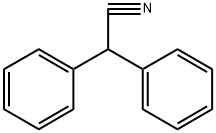
?????????
|
|
????????? ??
- ???
- 71-73 °C (lit.)
- ?? ?
- 181 °C/12 mmHg (lit.)
- ??
- 1.1061 (rough estimate)
- ???
- 21.3 hPa (190 °C)
- ???
- 1.5850 (estimate)
- ???
- 120 °C
- ?? ??
- Store below +30°C.
- ???
- ???
- ??? ??
- ??? ??
- ??
- ???? ??? ?? ??? ???
- ???
- ???
- Merck
- 14,3316
- BRN
- 1911160
- ?? ??
- NIOSH: IDLH 25 mg/m3
- InChIKey
- NEBPTMCRLHKPOB-UHFFFAOYSA-N
- LogP
- 2.795-3.197 at 25℃
- CAS ??????
- 86-29-3(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38 | ||
| ????? | 26-36-37/39 | ||
| WGK ?? | 2 | ||
| RTECS ?? | AL9800000 | ||
| TSCA | Yes | ||
| HS ?? | 29269095 | ||
| ?? | LD50 orally in Rabbit: 3500 mg/kg |
????????? C??? ??, ??, ??
??? ??
Diphenylacetonitrile is a white to creamy or faint yellow crystalline powder, Soluble in ethanol, ether. It is obtained by bromination and condensation of phenylacetonitrile. Diphenylacetonitrile is mainly used as an intermediate to manufacture API which deals in treatment of respiratory stimulant. It is used to manufacture APIs like stomach amine, Aminepentamide Sulphate, Diphenoxylate, Diphenylacetaldehyde, Doxapram, Loperamide, Methadone.??
Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceuticals.Diphenylacetonitrile is used to synthesize isocyanate, which is further prepared into UV paint, PU paint, transparent elastomer and adhesive, etc. In addition, it is also used in polyamide and epoxy resin industries.
?? ??
Diphenylacetonitrile can be prepared in good yield by the dehydration of the amide of diphenyl acetic acid with phosphorous oxychloride. Diphenylacetonitrile undergoes anhydrous condensation with ethyl-4-bromo-butyrate. This is followed by its hydrolysis in alkaline medium that is used in the quantitative determination of 3-cyano-3,3-diphenylpropionic acid.?? ??
In a 100 mL, nitrogen-flushed pear flask was added indium(III) bromide (2.0 mmol, 10 mol %) in CH2Cl2 (40 mL) to give a white suspension. To this at room temperature was added trimethylsilyl cyanide (40 mmol, 2.0 equiv.), followed by dropwise addition of alcohol (20 mmol, 1.0 equiv.) to give a moderate exothermic reaction. The mixture slowly turned clear, and TLC showed mostly the desire product. Saturated NaHCO3 was added and the mixture was concentrated in vacuo to remove the volatiles. The residue was partitioned between saturated NaHCO3 and ethyl acetate, and the aqueous layer was extracted one more time with ethyl acetate. Combined organic layers were dried over MgSO4, filtered and concentrated. The crude product was purified by flash column chromatography on silica gel (5% ethyl acetate/hexanes) to give the desired Diphenylacetonitrile.?? ??
Diphenylacetonitrile can be prepared in good yield by the dehydration of the amide of diphenyl acetic acid with phosphorous oxychloride.Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Moderately toxic by subcutaneous route. Questionable carcinogen with experimental carcinogenic and tumorigenic data. When heated to decomposition it emits toxic fumes of NOx, and CN-. See also NITRILES.Purification Methods
Crystallise the nitrile from EtOH or pet ether (b 90-100o). [Beilstein 9 H 674, 9 IV 2505.]????????? ?? ?? ? ???
???
?? ??
????????? ?? ??
???( 591)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| RongNa Biotechnology Co.,Ltd | +86-86-13583358881 +8618560316533 |
Brad@rongnabiotech.com | China | 3084 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3049 | 58 |
| Sinopharm International Guangzhou Ltd. | +86-2038033341 +86-18520036739 |
zhuluqiang@sinopharm.com | China | 76 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-18033737140 +86-17354101231 |
sales1@hbganmiao.com | China | 227 | 58 |
| Wuhan Ruichi Technology Co., Ltd | +8613545065237 |
admin@whrchem.com | China | 153 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 |
mina@chuanghaibio.com | China | 18137 | 58 |
| Wuhan Circle Star Chem-medical Technology Co.,Ltd | 027-85558552 +undefined13437180835 |
Janel@circlestar-chem.com | China | 1000 | 58 |
| Chongqing Soarwin Technology Co., Ltd | +86-19132938950 +86-19132938950 |
crystal@sorawin.cn | China | 360 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3883 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5868 | 58 |
????????? ?? ??:
?? ??? ????????? ?????????
XANTHENE-9-CARBONITRILE
2-(4-Amino-2-chloro-5-methylphenyl)-2-(4-chlorophenyl)acetonitrile
4-AMINO-2-CHLORODIPHENYLACETONITRILE
2,2-Diphenylpropionitrile
(4-AMINOPHENYL)PHENYLACETONITRILE HYDROCHLORIDE,97%
DIPHENOXYLATE HYDROCHLORIDE
Diphenoxylate
4-BROMO-2,2-DIPHENYLBUTYRONITRILE
4-DIPHENYLACETONITRILE
1,4-Phenylenediacetonitrile
1,2-Bis(cyanomethyl)benzene
2-[CYANO(PHENYL)METHYL]-6-FLUOROBENZENECARBONITRILE
2-(4-fluorophenyl)-2-phenyl-acetonitrile
2-[CYANO(4-FLUOROPHENYL)METHYL]-6-FLUOROBENZENECARBONITRILE