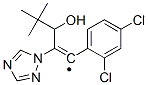
Diniconazole
|
|
Diniconazole ??
- ???
- 134~156℃
- ?? ?
- 501.1±60.0 °C(Predicted)
- ??
- 1.32
- ???
- 2.93 x l0-3 Pa (20 °C)
- ?? ??
- Inert atmosphere,Room Temperature
- ???
- DMSO:100.0(Max Conc. mg/mL);306.54(Max Conc. mM)
Water:0.67(Max Conc. mg/mL);2.05(Max Conc. mM)
- ??? ??
- ??
- ???
- 4mg l-1(25°C)
- ?? ?? (pKa)
- 12.89±0.20(Predicted)
- ??
- White to off-white
- CAS ??????
- 83657-24-3(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ?? | LD50 in male, female rats (mg/kg): 639, 474 orally; >5000, >5000 dermally (Takano) | ||
|---|---|---|---|
| ???? ?? | KE-10185 |
Diniconazole C??? ??, ??, ??
??
Diniconazole is used to control leaf and ear diseases in cereals, powdery mildew in vines, rust and black spot in roses, leaf spot in peanuts, Sigatoka disease in bananas, and Uredinales in coffee. It is also used on fruit, vegetables and ornamentals.?? ?? ??
Diniconazole is a mixture of four isomers, which are derived from a chiral carbon atom and a double bond in the molecule. Diniconazole M [( E)-( S)-1-(2 ,4-dichlorophenyl)-4,4-dimethyl-2-1(H -1,2,4-triazol-l-yl)pent- 1-en-3-ol] is more potent than diniconazole. The major metabolic pathways of diniconazole involve oxidative attack on the pentenol side chain to give products which may form conjugates. In mammals, sulfurcontaining metabolites may be formed.Degradation
Diniconazole is stable to heat, light and moisture. It undergoes photolysis and irradiation of a solution in methanol with light from a high-pressure mercury lamp for 8 hours gave three major and seven minor products, 2- 11 (Scheme 1). The major photoproduct was identified as the 2-isomer (2). The other major products (5 and 6) were formed by photooxidation of the CHOH group to a carbonyl group.When diniconazole was irradiated as a thin film under ultraviolet light (254 nm), the major product was identified as the Z-isomer. On the surface of a sandy loam soil, under the same irradiation conditions rapid degradation occurred with the formation of 2, 5 and 6. A thin film of diniconazole applied to a glass plate degraded very slowly in the dark. When the plate was placed in the sunhght, 70% of the applied material disappeared after 5 days exposure and isomerisation occurred to give the Z-isomer (65%). The DT50 in sunlight was 2.5 days. Under ultraviolet light (254 mn), the Z-isomer was formed after 15 minutes irradiation, and after 5 hours 90% of the material had isomerised (Dureja and Walia, 1992).
Diniconazole ?? ?? ? ???
???
???????
????? ???
olefine ketone
?? ??
3-???-5-???-1,2,4-????
??????????
3,3-Dimethyl-1-(1,2,4-triazole-1-yl)-2-butanone
2,4-??????????
?? ??
Diniconazole ?? ??
???( 246)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8805 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12830 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5888 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29871 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49374 | 58 |