4-??????? C??? ??, ??, ??
??? ??
beige crystalline solid. soluble in alcohol, ether and boiling petroleum ether, slightly soluble in cold petroleum ether, insoluble in water.
??
4-Nitroanisole is an intermediate in the manufacture of azo dyes. A toxic organic pollutant and a risk factor for urinary bladder cancer in humans.
??
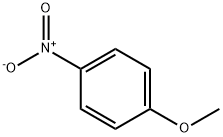
ChEBI: 4-nitroanisole is a member of the class of 4-nitroanisoles that is anisole in which one the hydrogen meta to the methoxy group is replaced by a nitro group.
?? ??
Produced from p-nitrochlorobenzene by methoxylation.
?? ??
A light red or amber-colored liquid or crystals. Insoluble in water and denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation or skin absorption. Used to make other chemicals.
??? ?? ??
Insoluble in water.
?? ???
4-Nitroanisole reacts explosively with (sodium hydroxide + zinc). 4-Nitroanisole reacts vigorously with hydrogen + catalyst (at 482° F and 25500 mm Hg).
????
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
????
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
A poison by ingestion.
Mutation data reported. Can explode in
presence of Ni. When heated to
decomposition it emits toxic fumes of NOx.
See also o-NITROANISOLE and NITRO
COMPOUNDS OF AROMATIC
HYDROCARBONS.
Purification Methods
Crystallise it from pet ether or hexane and dry it in vacuo. [Beilstein 6 IV 1282.]
4-??????? ?? ?? ? ???
???
?? ??