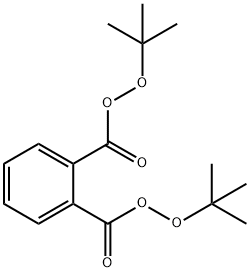
di-tert-butyl diperoxyphthalate
- CAS No.
- 2155-71-7
- Chemical Name:
- di-tert-butyl diperoxyphthalate
- Synonyms
- tert-Butyl diperphthalate;Di-tert-butyl peroxyphthalate;di-tert-butyl diperoxyphthalate;Diperoxyphthalic acid di-tert-butyl ester;1,2-Benzenedicarboperoxoic acid di(tert-butyl);1,2-Benzenediperoxycarboxylic acid di(tert-butyl) ester;Benzene-1,2-di(peroxycarboxylic acid)di-tert-butyl ester;1,2-Benzenedicarboperoxoic acid, 1,2-bis(1,1-dimethylethyl) ester
- CBNumber:
- CB5879485
- Molecular Formula:
- C16H22O6
- Molecular Weight:
- 310.34228
- MDL Number:
- MFCD00048239
- MOL File:
- 2155-71-7.mol
| Boiling point | 370.51°C (rough estimate) |
|---|---|
| Density | 1.1625 (rough estimate) |
| refractive index | 1.4600 (estimate) |
| EPA Substance Registry System | 1,2-Benzenedicarboperoxoic acid, 1,2-bis(1,1-dimethylethyl) ester (2155-71-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H315-H335 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
di-tert-butyl diperoxyphthalate Chemical Properties,Uses,Production
Chemical Properties
This peroxide powder with 8.45% active oxygen and 82% purity was mixed 20% in water and showed small pressure rises in the time–pressure assay when using 1 g of igniter. This indicates that this material poses a deflagration hazard.
General Description
Crystalline solid mixed with water. Water lessens the explosion hazard.
Reactivity Profile
Peroxides, such as DI-(TERT-BUTYLPEROXY)PHTHALATE, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness.
Purification Methods
Crystallise the perphthalate from Et2O or pet ether and dry it over H2SO4. The IR has max 1772cm-1 in CCl4. [Milas & Surgemor J Am Chem Soc 68 642 1946, Milas & Kelin J Org Chem 36 2900 1971, Beilstein 9 III 4190, 9 IV 3260.] Potentially EXPLOSIVE.
di-tert-butyl diperoxyphthalate Preparation Products And Raw materials
Raw materials
Preparation Products
di-tert-butyl diperoxyphthalate Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 | |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 | 1027@dideu.com | China | 10011 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 58 |