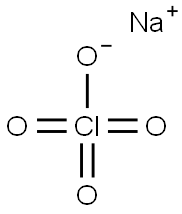Sodium perchlorate
- CAS No.
- 7601-89-0
- Chemical Name:
- Sodium perchlorate
- Synonyms
- NaClO4;Natriumperchlorat;SodiuM perchlorat;SODIUM PERCHLORATE;Natriumperchloraat;perchloratedesodium;sodio(percloratodi);sodium perchlolorate;KM sodium perchlorate;Perchlorate de sodium
- CBNumber:
- CB5242670
- Molecular Formula:
- ClNaO4
- Molecular Weight:
- 122.44037
- MDL Number:
- MFCD00003480
- MOL File:
- 7601-89-0.mol
| Melting point | 468 °C (lit.) |
|---|---|
| Boiling point | decomposes [HAW93] |
| Density | 2,2 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store at room temperature. |
| solubility | water: soluble2096g/L at 20°C |
| form | Liquid |
| color | White |
| Specific Gravity | 2.5 |
| PH | 6.0-8.0 (25℃, 5%) |
| Water Solubility | 209 G/100 ML (15 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8653 |
| Dielectric constant | 5.4(0.0℃) |
| Stability | Unstable. Strong oxidizer. Contact with combustible materials can cause fire. Shock sensitive and potentially explosive. Incompatible with a wide range of materials, including organics and other combustibles, powdered metals, acids, reducing agents. Very hygroscopic. Heat sensitive. |
| InChIKey | BAZAXWOYCMUHIX-UHFFFAOYSA-M |
| LogP | -7.18 at 20℃ |
| CAS DataBase Reference | 7601-89-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 97F4MTY3VA |
| NIST Chemistry Reference | Sodium perchlorate(7601-89-0) |
| EPA Substance Registry System | Perchloric acid, sodium salt (1:1) (7601-89-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H302-H319-H373 | |||||||||
| Precautionary statements | P210-P301+P312+P330-P305+P351+P338-P314 | |||||||||
| Hazard Codes | O,Xn | |||||||||
| Risk Statements | 9-22-48/22-36 | |||||||||
| Safety Statements | 13-22-27 | |||||||||
| RIDADR | UN 1502 5.1/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | SC9800000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28299000 | |||||||||
| Toxicity | LD50 orl-rat: 2100 mg/kg GTPZAB17(8),33,73 | |||||||||
| NFPA 704 |
|
Sodium perchlorate Chemical Properties,Uses,Production
Chemical Properties
Sodium perchlorate, NaCl04, is a flammable, white crystalline deliquescent solid that melts at 482 °C(899.6 °F). Soluble in water and alcohol, it is used as an analytical reagent. Sodium perchlorate is explosive in nature when in contact with concentrated sulfuric acid and finds use in explosives and jet fuels.
Uses
A chaotropic agent used in DNA extraction and hybridization
Uses
It is used in electrolyte; oxidimetric standard.
Uses
Sodium Perchlorate is an eluent component in the analysis of samples using liquid chromatography or high performance liquid chromatography. A chaotropic salt or a chaotropic agent. A useful raw material for batteries, plastics and in rubber products.
Definition
ChEBI: An inorganic sodium salt comprising equal numbers of sodium and perchlorate ions.
General Description
White crystalline solid. Noncombustible but will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Used in chemical analysis and in explosives.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium perchlorate is a strong oxidizing agent. Explosively decomposed at over 130°C [Merck 11th ed. 1989]. A mixture with powdered magnesium is a friction-sensitive mixture [Safety Eng. Reports 1947]. A mixture with ammonium nitrate is used as an explosive [Mellor 2 Supp. 1:608 1956]. A 1:1 mixture of the perchlorate and hydrazine exploded upon grinding, [Chem. Abs., 1969, 70, 53524].
Hazard
Dangerous fire and explosion risk in contact with organic materials and sulfuric acid.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A powerful oxidizer. Forms explosive mixture with acetone, 1,3-butylene glycol, 2,3butylene glycol, CaH2, charcoal, daminoethane, dimethyl formamide, ethanolamine, ethylene glycol, formamide, galactose, glycerin, hydrazine, water, NH4NO3, Mg, reducing agents, SrH2, urea. When heated to decomposition it emits toxic fumes of Cland NazO. See also PERCHLORATES.
Purification Methods
Because its solubility in water is high (2.1g/mL at 15o) and it has a rather low-temperature coefficient of solubility, sodium perchlorate is usually crystallised from acetone, MeOH, water/ethanol or dioxane/water (33g dissolved